Knockdown of BNST GluN2B-containing NMDA receptors mimics the actions of ketamine on novelty-induced hypophagia - PubMed (original) (raw)
Knockdown of BNST GluN2B-containing NMDA receptors mimics the actions of ketamine on novelty-induced hypophagia
K M Louderback et al. Transl Psychiatry. 2013.
Abstract
Administration of a single low dose of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine has been demonstrated to elicit long-lasting antidepressant effects in humans with depression, as well as in rodent models of depression. Although pharmacological studies have implicated the GluN2B subunit of the NMDA receptor in these effects, drugs targeting this subunit have off-target actions, and systemic administration of these compounds does not allow for delineation of specific brain regions involved. In this study, we assessed the role of GluN2B in the bed nucleus of the stria terminalis (BNST) in novelty-induced hypophagia (NIH) in mice. First, we verified that ketamine, as well as the GluN2B antagonist Ro25-6981, decreased the latency to consume food in a novel environment in a version of the NIH test. We then hypothesized that GluN2B-containing receptors within the BNST may be a target of systemic ketamine and contribute to behavioral effects. Through the combination of a GluN2B-floxed mouse line and stereotaxic delivery of lentiviral Cre recombinase, we found that targeted knockdown of this subunit within the BNST mimicked the reduction in affective behavior observed with systemic ketamine or Ro25-6981 in the NIH test. These data suggest a role for GluN2B-containing NMDARs within the BNST in the affective effects of systemic ketamine.
Figures
Figure 1
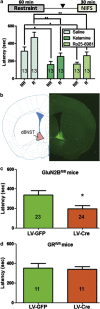
Viral deletion of GluN2B from the bed nucleus of the stria terminalis (BNST) phenocopies systemic treatment with ketamine or Ro25–6981. (a) Timeline above. Decreased latencies with systemic ketamine (3 mg kg−1) and Ro25 (5 mg kg−1) administration in our novelty-induced feeding suppression paradigm were repeated with (R) and without (NR) restraint stress. (b) Coronal atlas image (left) showing dorsal BNST in the inset and LV-GFP injection targeting (right). (c) Viral-mediated deletion of GluN2B from BNST (LV-Cre) phenocopies systemic ketamine; (d) however, viral-mediated deletion of GR from the BNST has no such effect. Data are presented as means with s.e.m. *_P_>0.05; **_P_>0.01. _N_s are indicated on bars.
Similar articles
- BNST GluN2D-Containing NMDA Receptors Influence Anxiety- and Depressive-like Behaviors and ModulateCell-Specific Excitatory/Inhibitory Synaptic Balance.
Salimando GJ, Hyun M, Boyt KM, Winder DG. Salimando GJ, et al. J Neurosci. 2020 May 13;40(20):3949-3968. doi: 10.1523/JNEUROSCI.0270-20.2020. Epub 2020 Apr 10. J Neurosci. 2020. PMID: 32277042 Free PMC article. - Ketamine modulates catecholamine transmission in the bed nucleus of stria terminalis: The possible role of this region in the antidepressant effects of ketamine.
Cadeddu R, Jadzic D, Carboni E. Cadeddu R, et al. Eur Neuropsychopharmacol. 2016 Oct;26(10):1678-82. doi: 10.1016/j.euroneuro.2016.08.009. Epub 2016 Aug 25. Eur Neuropsychopharmacol. 2016. PMID: 27569123 - Altered GluN2B NMDA receptor function and synaptic plasticity during early pathology in the PS2APP mouse model of Alzheimer's disease.
Hanson JE, Pare JF, Deng L, Smith Y, Zhou Q. Hanson JE, et al. Neurobiol Dis. 2015 Feb;74:254-62. doi: 10.1016/j.nbd.2014.11.017. Epub 2014 Dec 4. Neurobiol Dis. 2015. PMID: 25484285 Free PMC article. - An update on NMDA antagonists in depression.
Pochwat B, Nowak G, Szewczyk B. Pochwat B, et al. Expert Rev Neurother. 2019 Nov;19(11):1055-1067. doi: 10.1080/14737175.2019.1643237. Epub 2019 Jul 22. Expert Rev Neurother. 2019. PMID: 31328587 Review. - How does ketamine elicit a rapid antidepressant response?
Kavalali ET, Monteggia LM. Kavalali ET, et al. Curr Opin Pharmacol. 2015 Feb;20:35-9. doi: 10.1016/j.coph.2014.11.005. Epub 2014 Nov 25. Curr Opin Pharmacol. 2015. PMID: 25462290 Free PMC article. Review.
Cited by
- Delta Opioid Receptor-Mediated Antidepressant-Like Effects of Diprenorphine in Mice.
Olson KM, Hillhouse TM, Burgess GE, West JL, Hallahan JE, Dripps IJ, Ladetto AG, Rice KC, Jutkiewicz EM, Traynor JR. Olson KM, et al. J Pharmacol Exp Ther. 2023 Mar;384(3):343-352. doi: 10.1124/jpet.122.001182. Epub 2022 Dec 1. J Pharmacol Exp Ther. 2023. PMID: 36456196 Free PMC article. - BNST specific mGlu5 receptor knockdown regulates sex-dependent expression of negative affect produced by adolescent ethanol exposure and adult stress.
Kasten CR, Holmgren EB, Lerner MR, Wills TA. Kasten CR, et al. Transl Psychiatry. 2021 Mar 17;11(1):178. doi: 10.1038/s41398-021-01285-y. Transl Psychiatry. 2021. PMID: 33731684 Free PMC article. - Ketamine Blocks Morphine-Induced Conditioned Place Preference and Anxiety-Like Behaviors in Mice.
McKendrick G, Garrett H, Jones HE, McDevitt DS, Sharma S, Silberman Y, Graziane NM. McKendrick G, et al. Front Behav Neurosci. 2020 May 21;14:75. doi: 10.3389/fnbeh.2020.00075. eCollection 2020. Front Behav Neurosci. 2020. PMID: 32508606 Free PMC article. - BNST GluN2D-Containing NMDA Receptors Influence Anxiety- and Depressive-like Behaviors and ModulateCell-Specific Excitatory/Inhibitory Synaptic Balance.
Salimando GJ, Hyun M, Boyt KM, Winder DG. Salimando GJ, et al. J Neurosci. 2020 May 13;40(20):3949-3968. doi: 10.1523/JNEUROSCI.0270-20.2020. Epub 2020 Apr 10. J Neurosci. 2020. PMID: 32277042 Free PMC article. - Adolescent alcohol exposure produces sex differences in negative affect-like behavior and group I mGluR BNST plasticity.
Kasten CR, Carzoli KL, Sharfman NM, Henderson T, Holmgren EB, Lerner MR, Miller MC, Wills TA. Kasten CR, et al. Neuropsychopharmacology. 2020 Jul;45(8):1306-1315. doi: 10.1038/s41386-020-0670-7. Epub 2020 Apr 8. Neuropsychopharmacology. 2020. PMID: 32268346 Free PMC article.
References
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. - PubMed
MeSH terms
Substances
Grants and funding
- R01 MH079010/MH/NIMH NIH HHS/United States
- R37 AA019455/AA/NIAAA NIH HHS/United States
- R01 AA019455/AA/NIAAA NIH HHS/United States
- F31 AA021623/AA/NIAAA NIH HHS/United States
- T32 MH064913/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources