Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity - PubMed (original) (raw)
Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity
Denise E Lackey et al. Am J Physiol Endocrinol Metab. 2014 Feb.
Abstract
The extracellular matrix (ECM) plays an important role in the maintenance of white adipose tissue (WAT) architecture and function, and proper ECM remodeling is critical to support WAT malleability to accommodate changes in energy storage needs. Obesity and adipocyte hypertrophy place a strain on the ECM remodeling machinery, which may promote disordered ECM and altered tissue integrity and could promote proinflammatory and cell stress signals. To explore these questions, new methods were developed to quantify omental and subcutaneous WAT tensile strength and WAT collagen content by three-dimensional confocal imaging, using collagen VI knockout mice as a methods validation tool. These methods, combined with comprehensive measurement of WAT ECM proteolytic enzymes, transcript, and blood analyte analyses, were used to identify unique pathophenotypes of metabolic syndrome and type 2 diabetes mellitus in obese women, using multivariate statistical modeling and univariate comparisons with weight-matched healthy obese individuals. In addition to the expected differences in inflammation and glycemic control, approximately 20 ECM-related factors, including omental tensile strength, collagen, and enzyme transcripts, helped discriminate metabolically compromised obesity. This is consistent with the hypothesis that WAT ECM physiology is intimately linked to metabolic health in obese humans, and the studies provide new tools to explore this relationship.
Keywords: adipose inflammation; bariatric surgery; extracellular matrix; matrix metalloproteinase; type 2 diabetes mellitus.
Figures
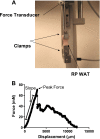
Fig. 1.
Apparatus for white adipose tissue (WAT) tensile strength measurement (A) and force/displacement curve example from murine retroperitoneal (RP) WAT (B). A DMT560 tissue puller with custom clamps used for measuring peak force of fresh murine RP WAT was used to measure slope, peak force, and tensile strength (see
methods
). Following peak force, the tissue begins to rupture, thus resulting in variable reductions in force/displacement, as illustrated.
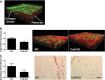
Fig. 2.
Mouse RP WAT collagen (extracellular matrix) content as measured by 5-(4,6-dichlorotriazinyl) aminofluorescein (5-DTAF) staining/confocal microscopy (A and B) and Sirius red histological staining (C): collagen is stained green (5-DTAF) and adipocytes are red (Bodipy 558/568 C12). In A, the starting image illustrating the collagen sheath typical of murine RP WAT surrounding the depot is depicted; to reduce variance and provide a more uniform method that could be applied to human WAT biopsies lacking a sheath, this layer was not included in the calculations shown in B. Values are presented as means ± SE comparing wild-type (WT) and collagen 6a1 knockout (Col6a) knockout mice. *Statistically significant at P < 0.05 by Student's _t_-test. Bar, 100 μm.
Fig. 3.
Healthy and metabolic syndrome (MetS) obese human adipocyte size distribution analysis. Adipocyte area was measured from Sirius red-stained paraffin sections of omental WAT (A) and subcutaneous (sc) WAT surgical biopsies (B). Bars indicate %total cells within the specified cell area range, shown as means ± SE. There were no statistically significant differences between healthy and MetS obese within the same size category, as determined by 1-way ANOVA followed by Tukey's post hoc test. Line in histology images indicates 100 μm at ×20 magnification.
Fig. 4.
Results from partial least squares-discriminant analysis (PLS-DA) modeling of clinical and WAT phenotype variables to identify features that differentiate healthy obese and MetS obese women. A: subjects scores plot illustrating individuals from the healthy (green) and MetS (pink) cohorts, showing separation of groups along latent variable 1 dimension (_x_-axis). B: scores separation along the _x_-axis dimension in A was explained primarily by variance in features depicted in the loadings plot. HOMA-IR, homeostasis model assessment of insulin resistance; BUN, blood urea nitrogren; ALT, alanine aminotransferase; CTSS, cathepsin S; ITGAX, integrin αX; MMP-7 and -9, matrix metalloproteinase-7 and -9, respectively; WBC, white blood cell; AST, aspartate aminotransferase; BP, blood pressure; ITGAD, integrin αD; BGN, biglycan; THBS1, thrombospondin-1; QUICKI, quantitative insulin sensitivity check index; ARG2, arginase 2; ECM, extracellular matrix.
Fig. 5.
Results from PLS-DA modeling of clinical and WAT phenotype variables to identify features that differentiate healthy obese and T2DM obese women. A: subjects scores plot illustrating individuals from the healthy (green) and MetS (red) cohorts, showing separation of groups along latent variable 1 dimension (_x_-axis). B: scores separation along the _x_-axis dimension in A was explained primarily by variance in features depicted in the loadings plot. Abbreviations are defined in tables and Fig. 4 legend.
Similar articles
- Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity.
Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, Langin D, Bedossa P, Zucker JD, Clement K. Henegar C, et al. Genome Biol. 2008 Jan 21;9(1):R14. doi: 10.1186/gb-2008-9-1-r14. Genome Biol. 2008. PMID: 18208606 Free PMC article. - Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity.
Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN, Thomas AP, Oort PJ, Kieffer DA, Amin R, Bettaieb A, Haj FG, Permana P, Anthony TG, Adams SH. Lackey DE, et al. Am J Physiol Endocrinol Metab. 2013 Jun 1;304(11):E1175-87. doi: 10.1152/ajpendo.00630.2012. Epub 2013 Mar 19. Am J Physiol Endocrinol Metab. 2013. PMID: 23512805 Free PMC article. - Healthy Subcutaneous and Omental Adipose Tissue Is Associated with High Expression of Extracellular Matrix Components.
Soták M, Rajan MR, Clark M, Biörserud C, Wallenius V, Hagberg CE, Börgeson E. Soták M, et al. Int J Mol Sci. 2022 Jan 4;23(1):520. doi: 10.3390/ijms23010520. Int J Mol Sci. 2022. PMID: 35008946 Free PMC article. - Omics Approaches in Adipose Tissue and Skeletal Muscle Addressing the Role of Extracellular Matrix in Obesity and Metabolic Dysfunction.
Anguita-Ruiz A, Bustos-Aibar M, Plaza-Díaz J, Mendez-Gutierrez A, Alcalá-Fdez J, Aguilera CM, Ruiz-Ojeda FJ. Anguita-Ruiz A, et al. Int J Mol Sci. 2021 Mar 9;22(5):2756. doi: 10.3390/ijms22052756. Int J Mol Sci. 2021. PMID: 33803198 Free PMC article. Review. - Contribution of adipogenesis to healthy adipose tissue expansion in obesity.
Vishvanath L, Gupta RK. Vishvanath L, et al. J Clin Invest. 2019 Oct 1;129(10):4022-4031. doi: 10.1172/JCI129191. J Clin Invest. 2019. PMID: 31573549 Free PMC article. Review.
Cited by
- Adipose tissue as an immunological organ.
Grant RW, Dixit VD. Grant RW, et al. Obesity (Silver Spring). 2015 Mar;23(3):512-8. doi: 10.1002/oby.21003. Epub 2015 Jan 22. Obesity (Silver Spring). 2015. PMID: 25612251 Free PMC article. Review. - Impact of Bariatric Surgery on Adipose Tissue Biology.
Osorio-Conles Ó, Vidal J, de Hollanda A. Osorio-Conles Ó, et al. J Clin Med. 2021 Nov 25;10(23):5516. doi: 10.3390/jcm10235516. J Clin Med. 2021. PMID: 34884217 Free PMC article. Review. - Adipose tissue depot-specific intracellular and extracellular cues contributing to insulin resistance in obese individuals.
Guzmán-Ruiz R, Tercero-Alcázar C, Rabanal-Ruiz Y, Díaz-Ruiz A, El Bekay R, Rangel-Zuñiga OA, Navarro-Ruiz MC, Molero L, Membrives A, Ruiz-Rabelo JF, Pandit A, López-Miranda J, Tinahones FJ, Malagón MM. Guzmán-Ruiz R, et al. FASEB J. 2020 Jun;34(6):7520-7539. doi: 10.1096/fj.201902703R. Epub 2020 Apr 15. FASEB J. 2020. PMID: 32293066 Free PMC article. - Microstructural inhomogeneity of electrical conductivity in subcutaneous fat tissue.
Kruglikov IL. Kruglikov IL. PLoS One. 2015 Mar 3;10(3):e0117072. doi: 10.1371/journal.pone.0117072. eCollection 2015. PLoS One. 2015. PMID: 25734656 Free PMC article. - EGR1 Transcription Factor is a Multifaceted Regulator of Matrix Production in Tendons and Other Connective Tissues.
Havis E, Duprez D. Havis E, et al. Int J Mol Sci. 2020 Feb 28;21(5):1664. doi: 10.3390/ijms21051664. Int J Mol Sci. 2020. PMID: 32121305 Free PMC article. Review.
References
- Abdennour M, Reggio S, La Naour G, Liu Y, Poitou C, Aron-Wisnewsky J, Charlotte F, Bouillot JL, Torcivia A, Sasso M, Miette V, Zucker JD, Bedossa P, Tordjman J, Clement K. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. In press - PubMed
- Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N, Tham JC, Welbourn R, Shore AC, Kos K, Winlove CP. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab 305: E1427–E1435, 2013 - PubMed
- Barbarroja N, López-Pedrera R, Mayas MD, García-Fuentes E, Garrido-Sánchez L, Macías-González M, El Bekay R, Vidal-Puig A, Tinahones FJ. The obese healthy paradox: is inflammation the answer? Biochem J 430: 141–149, 2010 - PubMed
- Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet 7: 2135–2140, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK-55758/DK/NIDDK NIH HHS/United States
- P20 RR021945/RR/NCRR NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- 8P20-GM-103528/GM/NIGMS NIH HHS/United States
- P20 GM103528/GM/NIGMS NIH HHS/United States
- 2P30-DK-072476/DK/NIDDK NIH HHS/United States
- R01-CA-112023/CA/NCI NIH HHS/United States
- P30 GM118430/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases