The pungent substances piperine, capsaicin, 6-gingerol and polygodial inhibit the human two-pore domain potassium channels TASK-1, TASK-3 and TRESK - PubMed (original) (raw)
The pungent substances piperine, capsaicin, 6-gingerol and polygodial inhibit the human two-pore domain potassium channels TASK-1, TASK-3 and TRESK
Leopoldo R Beltrán et al. Front Pharmacol. 2013.
Abstract
For a long time, the focus of trigeminal chemoperception has rested almost exclusively on TRP channels. However, two-pore domain (K2P) potassium channels have recently been identified as targets for substances associated with typical trigeminal sensations, such as numbing and tingling. In addition, they have been shown to be modulated by several TRP agonists. We investigated whether the pungent substances piperine, capsaicin, 6-gingerol and polygodial have an effect on human K2P channels. For this purpose, we evaluated the effects of these pungent substances on both wild-type and mutant K2P channels by means of two-electrode voltage-clamp experiments using Xenopus laevis oocytes. All four pungent substances were found to inhibit the basal activity of TASK-1 (K2P 3.1), TASK-3 (K2P 9.1), and TRESK (K2P 18.1) channels. This inhibitory effect was dose-dependent and, with the exception of polygodial on TASK-1, fully reversible. However, only piperine exhibited an IC50 similar to its reported EC50 on TRP channels. Finally, we observed for TASK-3 that mutating H98 to E markedly decreased the inhibition induced by piperine, capsaicin, and 6-gingerol, but not by polygodial. Our data contribute to the relatively sparse knowledge concerning the pharmacology of K2P channels and also raise the question of whether K2P channels could be involved in the pungency perception of piperine.
Keywords: 6-gingerol; K2P channels; capsaicin; piperine; polygodial; pungency.
Figures
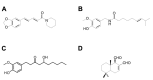
FIGURE 1
Chemical structure of (A) piperine, (B) capsaicin, (C) 6-gingerol and (D) polygodial.
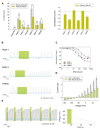
FIGURE 2
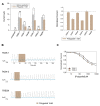
(A) Left (from left to right) absolute currents presented by: uninjected Xenopus oocytes, oocytes injected with cRNA coding for hTREK-1, hTASK-1, hTRAAK, hTASK-3, hTREK-2 and hTRESK, before and after the application of 300 μM piperine. The currents were registered at the final 50 ms of the +50 mV constant (see methods for a description of the ramp protocol used). Note that the amount of current carried by uninjected oocytes is negligible in comparison to oocytes expressing a K2P channel. Right. Normalized currents showing the effect of 300 μM piperine on Xenopus oocytes injected with cRNA coding for hTREK-1, hTASK-1, hTREK-1, hTASK-3, hTREK-2 and hTRESK. For each group, the current was normalized to the current registered prior to the application of 300 μM piperine (dotted line). All data are expressed as the mean +/- SEM. N of 3–6 for injected oocytes, N of 10 for uninjected oocytes. *P < 0.05, **P < 0.01, ***P < 0.001. (B) Representative voltage clamp recordings of Xenopus laevis oocytes expressing hTASK-1 (top), hTASK-3 (middle) and hTRESK (bottom), before, during and after the application of 100 μM piperine. In each case, piperine induced a fully reversible inhibition; for TASK-1, however, due to the prolonged recovery time, 40 ramps were not included. Dotted line represents zero current. (C) Dose-response curves for piperine on hTASK-1 (red circles), hTASK-3 (orange circles) and hTRESK (green circles). The IC50 values are listed in the text and in Table 1. N of 3-6 oocytes. For each measurement, the current was normalized to the current registered prior to the application of piperine. (D) hTASK-1 currents recorded before (gray bars) and after (green bars) the application of 300 μM piperine. The currents were elicited by voltage pulses from -140 to +80 mV in 20-mV steps, 500 ms duration from a holding potential of -80 mV. Values were obtained during the last 50 ms of the voltage pulses. N of 6 oocytes. (E) Left: representative voltage clamp recording of a Xenopus oocyte expressing hTASK-1 repetitively exposed to 30 μM piperine. Right: current clamp recording of a Xenopus oocyte expressing hTASK-1 exposed to 100 μM piperine, which led to a membrane depolarization of approximately 15 mV.
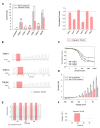
FIGURE 3
(A) Left (from left to right) absolute currents presented by: uninjected Xenopus oocytes, oocytes injected with cRNA coding for hTREK-1, hTASK-1, hTRAAK, hTASK-3, hTREK-2 and hTRESK, before and after the application of 100 μM capsaicin. Right: normalized currents showing the effect of 100 μM capsaicin on Xenopus oocytes injected with cRNA coding for hTREK-1, hTASK-1, hTRAAK, hTASK-3, hTREK-2 and hTRESK. For each group, the current was normalized to the current registered prior to the application of 100 μM capsaicin (dotted line). All data are expressed as the mean +/- SEM. N of 3–6 oocytes. *P < 0.05, **P < 0.01. (B) Representative voltage clamp recordings of Xenopus laevis oocytes expressing TASK-1 (top), TASK-3 (middle) and TRESK (bottom), before, during and after the application of 100 μM capsaicin, showing in every case a fully reversible inhibition. Dotted line represents zero current. (C) Dose-response curves for capsaicin on TASK-1 (red circles), TASK-3 (orange circles) and TRESK (green circles). The IC50 values are listed in the text and in Table 1. N of 3–6 oocytes. For each measurement, the current was normalized to the current registered prior to the application of capsaicin. (D) hTASK-3 currents recorded before and after the application of 100 μM capsaicin. The currents were elicited by voltage pulses from -140 to +80 mV in 20 mV steps, 500 ms duration from a holding potential of -80 mV. Values were obtained during the last 50 ms of the voltage pulses. N of 6 oocytes. (E) Representative voltage clamp recording of a Xenopus oocyte expressing TASK-3 repetitively exposed to 30 μM capsaicin. (F) Current clamp recording of a Xenopus oocyte expressing TASK-3 exposed to 100 μM capsaicin, which led to a membrane depolarization of approximately 30 mV.
FIGURE 4
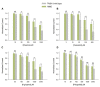
(A) Left (from left to right) absolute currents presented by: uninjected Xenopus oocytes, oocytes injected with cRNA coding for hTREK-1, hTASK-1, hTRAAK, hTASK-3, hTREK-2 and hTRESK, before and after the application of 300 μM 6-gingerol. Right normalized currents showing the effect of 300 μM 6-gingerol on Xenopus oocytes injected with cRNA coding for hTREK-1, hTASK-1, hTRAAK, hTASK-3, hTREK-2 and hTRESK. For each group, the current was normalized to the current registered prior to the application of 300 μM 6-gingerol (dotted line). All data are expressed as the mean +/- SEM. N of 9–18 oocytes. *P < 0.05, **P < 0.01, ***P < 0.001. (B) Representative voltage clamp recordings of Xenopus laevis oocytes expressing TASK-1 (top), TASK-3 (middle) and TRESK (bottom), before, during and after the application of 300 μM 6-gingerol, showing fully reversible inhibition in all cases. Dotted line represents zero current. (C) Dose-response curves for 6-gingerol on TASK-1 (red circles), TASK-3 (orange circles) and TRESK (green circles). N of 3–19 oocytes. For each measurement, the current was normalized to the current registered prior to the application of 6-gingerol.
FIGURE 5
(A) Left (from left to right) absolute currents presented by: uninjected Xenopus oocytes, oocytes injected with cRNA coding for hTREK-1, hTASK-1, hTRAAK, hTASK-3, hTREK-2 and hTRESK, before and after the application of 1 mM polygodial. Right. Normalized currents showing the effect of 1 mM polygodial on Xenopus oocytes injected with cRNA coding for hTREK-1, hTASK-1, hTRAAK, hTASK-3, hTREK-2 and hTRESK. For each group, the current was normalized to the current registered prior to the application of 1 mM polygodial (dotted line). All data are expressed as the mean +/- SEM. N of 3 to 6 oocytes. *P < 0.05. (B) Representative voltage clamp recordings of Xenopus laevis oocytes expressing TASK-1 (top), TASK-3 (middle) and TRESK (bottom), before, during and after the application of 1 mM polygodial, showing a partially reversible inhibition for TASK-1 and TASK-3. Dotted line represents zero current. (C) Dose-response curves for polygodial on TASK-1 (red circles) and TASK-3 (orange circles). The IC50 values are listed in the text and in Table 1. N of 3–5 oocytes. For each measurement, the current was normalized to the current registered prior to the application of polygodial.
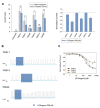
FIGURE 6
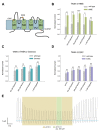
(A) Human TASK-3 membrane topology diagram. Four transmembrane segments (M1 to M4) and two pore regions (P1 and P2) are shown. Mutated amino acids are indicated either with a star or as the replaced sequence. (B) Comparative effects of pH 5.0, 100 μM capsaicin, 300 μM piperine, 1 mM 6-gingerol and 1 mM polygodial on wild-type human TASK-3 (gray bars) and the H98E mutant (green bars). N of 4–6 oocytes. *P < 0.05, **P < 0.01, ***P < 0.001. (C) Comparative effects of the four pungent substances under evaluation in wild-type hTASK-3 (gray bars) vs. TREK-2 chimera (light blue bars). N of 4-5 oocytes. (D) Comparative effects of pH 5.0, 100 μM capsaicin, 300 μM piperine, 1 mM 6-gingerol and 1 mM polygodial on wild-type human TASK-3 (gray bars) and the E30C mutant (purple bars). N of 4–6 oocytes. (E) Representative voltage clamp recordings of a Xenopus laevis oocyte expressing TASK-3 WT exposed to a pH of 6.5 and then to 300 μM piperine.
FIGURE 7
Dose response relationships for the inhibition induced by pungent substances on TASK-3 WT (gray bars) vs. H98E (green bars) for (A) piperine, N of 5–12 oocytes; (B) capsaicin, N of 5–10 oocytes; (C) 6-gingerol, N of 3–19 oocytes and (D) polygodial N of 3–9 oocytes. *P < 0.05, **P < 0.01, ***P < 0.001.
Similar articles
- The Effect of Pungent and Tingling Compounds from Piper nigrum L. on Background K+ Currents.
Beltrán LR, Dawid C, Beltrán M, Levermann J, Titt S, Thomas S, Pürschel V, Satalik M, Gisselmann G, Hofmann T, Hatt H. Beltrán LR, et al. Front Pharmacol. 2017 Jun 26;8:408. doi: 10.3389/fphar.2017.00408. eCollection 2017. Front Pharmacol. 2017. PMID: 28694780 Free PMC article. - Atrial fibrillation and heart failure-associated remodeling of two-pore-domain potassium (K2P) channels in murine disease models: focus on TASK-1.
Wiedmann F, Schulte JS, Gomes B, Zafeiriou MP, Ratte A, Rathjens F, Fehrmann E, Scholz B, Voigt N, Müller FU, Thomas D, Katus HA, Schmidt C. Wiedmann F, et al. Basic Res Cardiol. 2018 Jun 7;113(4):27. doi: 10.1007/s00395-018-0687-9. Basic Res Cardiol. 2018. PMID: 29881975 - Regulation of two-pore-domain (K2P) potassium leak channels by the tyrosine kinase inhibitor genistein.
Gierten J, Ficker E, Bloehs R, Schlömer K, Kathöfer S, Scholz E, Zitron E, Kiesecker C, Bauer A, Becker R, Katus HA, Karle CA, Thomas D. Gierten J, et al. Br J Pharmacol. 2008 Aug;154(8):1680-90. doi: 10.1038/bjp.2008.213. Epub 2008 Jun 2. Br J Pharmacol. 2008. PMID: 18516069 Free PMC article. - Molecular Pharmacology of K2P Potassium Channels.
Decher N, Rinné S, Bedoya M, Gonzalez W, Kiper AK. Decher N, et al. Cell Physiol Biochem. 2021 Mar 6;55(S3):87-107. doi: 10.33594/000000339. Cell Physiol Biochem. 2021. PMID: 33667333 Review. - Two-pore domain potassium channels: potential therapeutic targets for the treatment of pain.
Mathie A, Veale EL. Mathie A, et al. Pflugers Arch. 2015 May;467(5):931-43. doi: 10.1007/s00424-014-1655-3. Epub 2014 Nov 26. Pflugers Arch. 2015. PMID: 25420526 Review.
Cited by
- Stochastic Ultralow-Frequency Oscillations of the Luminescence Intensity from the Surface of a Polymer Membrane Swelling in Aqueous Salt Solutions.
Bunkin NF, Bolotskova PN, Bondarchuk EV, Gryaznov VG, Kozlov VA, Okuneva MA, Ovchinnikov OV, Smoliy OP, Turkanov IF, Galkina CA, Dmitriev AS, Seliverstov AF. Bunkin NF, et al. Polymers (Basel). 2022 Feb 11;14(4):688. doi: 10.3390/polym14040688. Polymers (Basel). 2022. PMID: 35215601 Free PMC article. - Clinical concentrations of chemically diverse general anesthetics minimally affect lipid bilayer properties.
Herold KF, Sanford RL, Lee W, Andersen OS, Hemmings HC Jr. Herold KF, et al. Proc Natl Acad Sci U S A. 2017 Mar 21;114(12):3109-3114. doi: 10.1073/pnas.1611717114. Epub 2017 Mar 6. Proc Natl Acad Sci U S A. 2017. PMID: 28265069 Free PMC article. - Chemogenic Subqualities of Mouthfeel.
Simons CT, Klein AH, Carstens E. Simons CT, et al. Chem Senses. 2019 May 29;44(5):281-288. doi: 10.1093/chemse/bjz016. Chem Senses. 2019. PMID: 31039245 Free PMC article. Review. - Potent and selective inhibitors of the TASK-1 potassium channel through chemical optimization of a bis-amide scaffold.
Flaherty DP, Simpson DS, Miller M, Maki BE, Zou B, Shi J, Wu M, McManus OB, Aubé J, Li M, Golden JE. Flaherty DP, et al. Bioorg Med Chem Lett. 2014 Aug 15;24(16):3968-73. doi: 10.1016/j.bmcl.2014.06.032. Epub 2014 Jun 19. Bioorg Med Chem Lett. 2014. PMID: 25017033 Free PMC article. - Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice.
Sałat K, Filipek B. Sałat K, et al. J Zhejiang Univ Sci B. 2015 Mar;16(3):167-78. doi: 10.1631/jzus.B1400189. J Zhejiang Univ Sci B. 2015. PMID: 25743118 Free PMC article.
References
- Barnes C. S., Loder J. W. (1962). The structure of polygodial: a new sesquiterpene dialdehyde from polygonum hydropiper L. Austr. J. Chem. 15 322–327 10.1071/CH9620322 - DOI
- Belitz H. D., Grosch W., Schieberle P. (2004). Food Chemistry 3rd Edn Berlin: Springer Verlag; 10.1007/978-3-662-07279-0 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources