Development, maintenance and disruption of the blood-brain barrier - PubMed (original) (raw)
Review
. 2013 Dec;19(12):1584-96.
doi: 10.1038/nm.3407. Epub 2013 Dec 5.
Affiliations
- PMID: 24309662
- PMCID: PMC4080800
- DOI: 10.1038/nm.3407
Review
Development, maintenance and disruption of the blood-brain barrier
Birgit Obermeier et al. Nat Med. 2013 Dec.
Abstract
The interface between the blood circulation and the neural tissue features unique characteristics that are encompassed by the term 'blood-brain barrier' (BBB). The main functions of this barrier, namely maintenance of brain homeostasis, regulation of influx and efflux transport, and protection from harm, are determined by its specialized multicellular structure. Every constituent cell type makes an indispensable contribution to the BBB's integrity. But if one member of the BBB fails, and as a result the barrier breaks down, there can be dramatic consequences and neuroinflammation and neurodegeneration can occur. In this Review, we highlight recently gained mechanistic insights into the development and maintenance of the BBB. We then discuss how BBB disruption can cause or contribute to neurological disease. Finally, we examine how this knowledge can be used to explore new possibilities for BBB repair.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
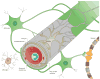
Figure 1. Cellular interplay at the neurovascular unit (capillary level)
The blood-brain barrier (BBB) is part of the neurovascular unit (NVU), which represents an elaborate interplay of central and peripheral cells. Vascular endothelial cells sealed by tight junctions constitute the BBB. The endothelium’s abluminal surface is covered by a basement membrane in which pericytes and their processes are embedded. Direct intercellular crosstalk between endothelial cells and pericytes are implemented by peg-socket junctions. Astrocytes extend foot processes which encircle the abluminal side of the vessel to an extent of nearly 100%. Although at the capillary level the basement membrane is regarded as a composite basement membrane, it is separated into endothelial and parenchymal basement membranes at the level of the post-capillary venule, delimiting the perivascular space (not shown). Neurons and microglia are considered members of the NVU as they interact with core elements of the BBB and influence barrier functions. Peripheral blood cells including leukocytes also participate in this cellular interplay as they modulate BBB functions under pathological conditions such as inflammation.
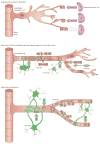
Figure 2. Major signaling pathways in BBB development
a) Development of the BBB begins with angiogenesis when endothelial progenitor cells invade the embryonic neuroectoderm. Neural progenitor cells secrete factors that guide sprouting endothelial cells. Vascular endothelial growth factor (Vegf) serves as a cue for endothelial cells which express the receptor Flk-1. Neural progenitor secreted Wnt ligands bind Frizzled (Fzd) receptors on the endothelium which is required for migration of endothelial cells into the embryonic neural tissue. Wnt signaling also leads to the transcription of BBB-related genes including those encoding Glut-1 and tight junction (TJ) molecules. Angiogenic sprouting requires the endothelial orphan receptor Gpr124, which regulates the migration of endothelial cells and expression of Glut-1. b) The second major stage of BBB development is characterized by the investment in endothelial cells by pericytes and astrocytes, which promote barrier properties in the cerebral endothelial cells. Endothelial cells of nascent vessels release platelet-derived growth factor-b (Pdgf-b) and thereby recruit pericytes that express the receptor Pdgfr-β to the endothelial surface. Interactions between endothelial cells and pericytes are mediated by bidirectional transforming growth factor-β (Tgf-β)-TGF-β receptor (Tgf-βR) signaling, leading to two major effects. First, upregulation of endothelial Cadherin-2 leads to firm adhesion between endothelial cells and pericytes, and second, pericytes are stimulated to deposit extracellular matrix (ECM) components, contributing to basement membrane formation. When pericytes are set in place, they limit BBB permeability by producing Ang-1 that signals to endothelial Tie-2. Astrocytes are involved in limiting BBB permeability by the release of Sonic Hedgehog (Shh), which activates Hh signaling in endothelial cells through the receptor Patched-1 (Ptc1). Furthermore, activated Src-suppressed C-kinase substrate (SSeCKS) in astrocytes stimulates Ang-1 production, which signals back to endothelial Tie-2 receptors. These interactions subsequently lead to the development of more advanced TJs, loss of leukocyte adhesion molecules and inhibition of transcytosis. c) Sealing of interendothelial TJs by upregulation and redistribution of TJ proteins is completed during maturation and needs to be maintained. Wnt ligands from an unknown progenitor and astrocytes regulate TJ formation through the Fzd receptor expressed by endothelial cells. Crosstalk between endothelial cells and pericytes mediated by TGF-β-TGF-βR and Ang-1-Tie-2 signaling supports BBB formation and maintenance. Sustained BBB integrity is mainly implemented by astrocytes. Apolipoprotein E (Apoe) produced by astrocytes signals through lipoprotein receptor-related protein 1 (Lrp-1) on brain microvessels. It has also been hypothesized that astrocytic Apoe acts on pericytes, which in turn regulate endothelial TJs (not shown). Endothelial cells upregulate TJ protein expression after activation by Shh produced by astrocytes. Astrocyte-derived angiotensin (Ang) binds to AT1 receptors on endothelial cells and promotes the formation and maintenance of interendothelial TJs. Ligands and receptors are colored according to the cell type of origin: neural progenitor cell, purple; endothelial cell, red; pericyte, beige; astrocyte, green; unknown, white; microvessel, grey;
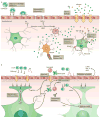
Figure 3. Central role of pericytes in the neurovascular unit
Pericytes interact with and influence various members of the neurovascular unit. They promote development of the BBB by supporting sprouting, differentiation, and maturation of endothelial cells. The interaction of endothelial cells with pericytes induces tight junction formation. The basement membrane is partially built by pericytes, which contribute components that regulate BBB development and maintenance. Under inflammatory conditions, pericytes stimulate immune cells to produce cytokines and to present antigens. Pericytes may guide astrocyte end-foot processes towards endothelial tubes and initiate their polarization. Pericytes also support proper neuronal functions.
Figure 4. Causes, characteristics and consequences of BBB breakdown
Factors that can disrupt the BBB are varied, ranging from secreted elements to immune cells and pathogens. Compromised BBB integrity manifests mainly as increased barrier permeability. In addition to direct effects on endothelial cells, other members of the neurovascular unit can be affected, that is pericytes, astrocytes and basement membrane, which in turn aggravate impairment of BBB functions. Consequences vary from dysregulated molecular and ionic flux across the damaged BBB to the initiation of a central inflammatory response. Despite manifold causes, characteristics and consequences, BBB breakdown generally culminates in neuronal dysfunction, neuroinflammation, and neurodegeneration. Downstream pathological outcomes and potential for recovery are diverse. ROS, reactive oxygen species; MMPs, matrix metalloproteinases
Figure 5. Pathogenic mechanisms of epilepsy and neuromyelitis optica
a) Epileptic seizures can be promoted by luminal leukocyte-endothelial interactions, allowing plasma K+ to enter the CNS, which lowers the threshold for seizures. Epileptogenic inflammation during and after seizures is sustained by astrocytes that release cytokines and chemokines (for example, IL6 and CCL2). Microglia and astrocytes produce IL-1β and VEGF, resulting in increased BBB permeability by downregulation of endothelial ZO-1. A compromised BBB leads to leakage of plasma components across the endothelial cell monolayer. Increased levels of K+ and Glu enhance neuron excitability. Extravasated albumin is taken up by astrocytes via TGF-βR and leads to Smad2-mediated downregulation of the K+ channel Kir4.1, decreased expression of Glu transporter EAAT-2 is initiated by astrocytic TNF-α. Both mechanisms exacerbate neuronal hyperactivity due to impaired K+ and Glu buffering by astrocytes. b) In NMO, lesions are characterized by loss of astrocytes, immunoglobulin and complement deposits, and neutrophil and eosinophil infiltrates. AQP4-IgG (gray) recognize AQP4 (gray triangles) on astrocytic end-feet. AQP4-IgG effector functions include: Binding of AQP4-IgG to its antigen leads to internalization and degradation of AQP4 in endosomes, which ultimately affects BBB function; AQP4-IgG induce astrocyte death by complement-dependent cytotoxicity (CDC) and/or antibody-dependent cellular cytotoxicity (ADCC). Activated complement anaphylatoxins and astrocyte-derived CCL5 and CXCL1 recruit eosinophils and neutrophils, which contribute to brain tissue damage and BBB breakdown by the production of ROS. Elevated CXCL8 levels trigger the secretion of MMP-9 by neutrophils, leading to basement membrane degradation. BBB permeability is further increased by activation of the BBB endothelium, possibly mediated by IgG (purple) binding to the vascular surface. As a result, endothelial ICAM-1 is upregulated and release of VEGF and TNF-α is initiated.
Similar articles
- The blood-brain barrier.
Obermeier B, Verma A, Ransohoff RM. Obermeier B, et al. Handb Clin Neurol. 2016;133:39-59. doi: 10.1016/B978-0-444-63432-0.00003-7. Handb Clin Neurol. 2016. PMID: 27112670 Review. - Mfsd2a is critical for the formation and function of the blood-brain barrier.
Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Ben-Zvi A, et al. Nature. 2014 May 22;509(7501):507-11. doi: 10.1038/nature13324. Epub 2014 May 14. Nature. 2014. PMID: 24828040 Free PMC article. - Blood-brain barrier interfaces and brain tumors.
Lee SW, Kim WJ, Park JA, Choi YK, Kwon YW, Kim KW. Lee SW, et al. Arch Pharm Res. 2006 Apr;29(4):265-75. doi: 10.1007/BF02968569. Arch Pharm Res. 2006. PMID: 16681030 Review. - Factors influencing the blood-brain barrier permeability.
Zhao Y, Gan L, Ren L, Lin Y, Ma C, Lin X. Zhao Y, et al. Brain Res. 2022 Aug 1;1788:147937. doi: 10.1016/j.brainres.2022.147937. Epub 2022 May 11. Brain Res. 2022. PMID: 35568085 Review. - The molecular, cellular, and morphological components of blood-brain barrier development during embryogenesis.
Hagan N, Ben-Zvi A. Hagan N, et al. Semin Cell Dev Biol. 2015 Feb;38:7-15. doi: 10.1016/j.semcdb.2014.12.006. Epub 2014 Dec 27. Semin Cell Dev Biol. 2015. PMID: 25550218 Review.
Cited by
- Passage of Magnetic Tat-Conjugated Fe3O4@SiO2 Nanoparticles Across In Vitro Blood-Brain Barrier.
Zhao X, Shang T, Zhang X, Ye T, Wang D, Rei L. Zhao X, et al. Nanoscale Res Lett. 2016 Dec;11(1):451. doi: 10.1186/s11671-016-1676-2. Epub 2016 Oct 10. Nanoscale Res Lett. 2016. PMID: 27726119 Free PMC article. - Optic nerve sheath diameter guided detection of sepsis-associated encephalopathy.
Suresh V. Suresh V. Crit Care. 2020 Aug 25;24(1):520. doi: 10.1186/s13054-020-03232-7. Crit Care. 2020. PMID: 32843090 Free PMC article. No abstract available. - Hemorrhagic stroke-induced subtype of inflammatory reactive astrocytes disrupts blood-brain barrier.
Liu C, Guo Y, Deng S, Zhou S, Wu S, Chen T, Shi X, Mamtilahun M, Xu T, Liu Z, Li H, Zhang Z, Tian H, Chung WS, Wang J, Yang GY, Tang Y. Liu C, et al. J Cereb Blood Flow Metab. 2024 Jul;44(7):1102-1116. doi: 10.1177/0271678X241235008. Epub 2024 Feb 22. J Cereb Blood Flow Metab. 2024. PMID: 38388375 - Hypothalamic Microglial Heterogeneity and Signature under High Fat Diet-Induced Inflammation.
Mendes NF, Jara CP, Zanesco AM, de Araújo EP. Mendes NF, et al. Int J Mol Sci. 2021 Feb 24;22(5):2256. doi: 10.3390/ijms22052256. Int J Mol Sci. 2021. PMID: 33668314 Free PMC article. Review. - A Norrin/Wnt surrogate antibody stimulates endothelial cell barrier function and rescues retinopathy.
Chidiac R, Abedin M, Macleod G, Yang A, Thibeault PE, Blazer LL, Adams JJ, Zhang L, Roehrich H, Jo HN, Seshagiri S, Sidhu SS, Junge HJ, Angers S. Chidiac R, et al. EMBO Mol Med. 2021 Jul 7;13(7):e13977. doi: 10.15252/emmm.202113977. Epub 2021 Jun 9. EMBO Mol Med. 2021. PMID: 34105895 Free PMC article.
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience. 2006;7:41–53. - PubMed
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. - PubMed
- Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72:648–672. - PubMed
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circulation research. 2007;100:158–173. - PubMed
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circulation research. 2007;100:174–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical