Epigenetic events in liver cancer resulting from alcoholic liver disease - PubMed (original) (raw)
Review
Epigenetic events in liver cancer resulting from alcoholic liver disease
Samuel W French. Alcohol Res. 2013.
Abstract
Epigenetic mechanisms play an extensive role in the development of liver cancer (i.e., hepatocellular carcinoma [HCC]) associated with alcoholic liver disease (ALD) as well as in liver disease associated with other conditions. For example, epigenetic mechanisms, such as changes in the methylation and/or acetylation pattern of certain DNA regions or of the histone proteins around which the DNA is wrapped, contribute to the reversion of normal liver cells into progenitor and stem cells that can develop into HCC. Chronic exposure to beverage alcohol (i.e., ethanol) can induce all of these epigenetic changes. Thus, ethanol metabolism results in the formation of compounds that can cause changes in DNA methylation and interfere with other components of the normal processes regulating DNA methylation. Alcohol exposure also can alter histone acetylation/deacetylation and methylation patterns through a variety of mechanisms and signaling pathways. Alcohol also acts indirectly on another molecule called toll-like receptor 4 (TLR4) that is a key component in a crucial regulatory pathway in the cells and whose dysregulation is involved in the development of HCC. Finally, alcohol use regulates an epigenetic mechanism involving small molecules called miRNAs that control transcriptional events and the expression of genes important to ALD.
Figures
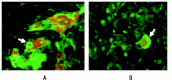
Figure 1
Histone deacetylase 1 (HDAC1) is upregulated in the nuclei of liver cells (i.e., hepatocytes) that form Mallory-Denk bodies (MDBs), which are indicative of liver damage. The image shown is from a liver biopsy from a patient with alcoholic hepatitis. The liver section was IHC double stained for HDAC1 (green nuclei arrows) (A), ubiquitin to identify cells with MDBs (red, arrows) (B), and tricolor (C). Magnification: ×350.
Figure 2
The signaling molecule p27 is upregulated in the nuclei of liver cells (i.e., hepatocytes) in a liver biopsy from two patients with alcoholic hepatitis. The livers were stained with an immunoperoxidase-labeled antibody that recognizes p27. The hepatocyte nuclei positive for p27 appear brown; those that are negative for p27 appear blue. (A and B) Most of the nuclei stained positive. (B) The Mallory-Denk bodies (MDBs) also stained brown (arrows), indicating that p27 also is sequestered in the MDBs. Magnification ×520.
Figure 3
These images show a double-immunostained liver biopsy from a patient with alcoholic hepatitis where most of the hepatocytes had formed Mallory-Denk bodies (MDBs). The MDBs stained positive for (A) pEZHZ (green), (B) ubiquitin (red), and (C) merged (yellow), indicating that the pEZH2 colocalized in the MDBs. Magnification ×350.
Figure 4
Analysis of different marker proteins in stem cell/progenitor cells located in the livers of patients with alcoholic liver disease with cirrhosis and associated hepatocellular carcinoma (HCC). (A) Liver cirrhosis and HCC samples stained for both YAP-1 (green) and IGF2bp3 (red). a) Cirrhosis; b) HCC (magnification ×350); c) HCC; d) HCC; e) Tricolor image merged from c and d (magnification ×525). (B), a and b) Liver cirrhosis sample double stained for Nanog (green) and SOX2 (red). Note the Mallory-Denk bodies (MDBs) (arrow) stain positive for SOX 2. c and d) Liver cells stained for Yap 1 (green) and SOX 2 (red). The liver cells/progenitor cells stain positive for SOX2 (arrows). Magnification ×780. (C) Liver sample from a patient with alcoholic hepatitis double stained for the Nanog protein (green) and ubiquitin (red). The stem cell stains positive for Nanog (pink arrow) and an MDB stained positive for ubiquitin (white arrow). Magnification ×780.
Figure 5
Immunohistochemical analysis of a liver biopsy obtained from a patient with alcoholic hepatitis with Mallory-Denk body (MDB) formation. The samples were stained for the presence of CD49f (integrin subunit α6) (green) and ubiquitin (red). Note that the MDBs stain both red for ubiquitin and green for CD49f. The arrows point to the nuclei that stain green except for the nucleolus. The yellow fringe on the MDB indicates colocalization of both proteins at the interface of the MDBs. The round black holes are macrovesicular fat globules in the hepatocytes. A) (magnification ×700) shows a cluster of MDB-forming cells. B) (magnification ×1,050) shows a single cell forming an MDB.
Similar articles
- Epigenetic effects of ethanol on the liver and gastrointestinal system.
Shukla SD, Lim RW. Shukla SD, et al. Alcohol Res. 2013;35(1):47-55. Alcohol Res. 2013. PMID: 24313164 Free PMC article. Review. - Epigenetic regulation in alcoholic liver disease.
Mandrekar P. Mandrekar P. World J Gastroenterol. 2011 May 28;17(20):2456-64. doi: 10.3748/wjg.v17.i20.2456. World J Gastroenterol. 2011. PMID: 21633650 Free PMC article. Review. - PNPLA3 Gene Polymorphism Is Associated With Predisposition to and Severity of Alcoholic Liver Disease.
Salameh H, Raff E, Erwin A, Seth D, Nischalke HD, Falleti E, Burza MA, Leathert J, Romeo S, Molinaro A, Corradini SG, Toniutto P, Spengler U, Daly A, Day CP, Kuo YF, Singal AK. Salameh H, et al. Am J Gastroenterol. 2015 Jun;110(6):846-56. doi: 10.1038/ajg.2015.137. Epub 2015 May 12. Am J Gastroenterol. 2015. PMID: 25964223 Review. - A common polymorphism in the NCAN gene is associated with hepatocellular carcinoma in alcoholic liver disease.
Nischalke HD, Lutz P, Krämer B, Söhne J, Müller T, Rosendahl J, Fischer J, Berg T, Hittatiya K, Fischer HP, Soyka M, Semmo N, Nattermann J, Sauerbruch T, Strassburg CP, Stickel F, Spengler U. Nischalke HD, et al. J Hepatol. 2014 Nov;61(5):1073-9. doi: 10.1016/j.jhep.2014.06.006. Epub 2014 Jun 16. J Hepatol. 2014. PMID: 24946282 - MicroRNAs in alcoholic liver disease.
Szabo G, Satishchandran A. Szabo G, et al. Semin Liver Dis. 2015 Feb;35(1):36-42. doi: 10.1055/s-0034-1397347. Epub 2015 Jan 29. Semin Liver Dis. 2015. PMID: 25632933 Free PMC article. Review.
Cited by
- The Roles of Myeloid-Derived Suppressor Cells in Liver Disease.
Zhang C, Sui Y, Liu S, Yang M. Zhang C, et al. Biomedicines. 2024 Jan 27;12(2):299. doi: 10.3390/biomedicines12020299. Biomedicines. 2024. PMID: 38397901 Free PMC article. Review. - Glutathione and Transsulfuration in Alcohol-Associated Tissue Injury and Carcinogenesis.
Chen Y, Han M, Matsumoto A, Wang Y, Thompson DC, Vasiliou V. Chen Y, et al. Adv Exp Med Biol. 2018;1032:37-53. doi: 10.1007/978-3-319-98788-0_3. Adv Exp Med Biol. 2018. PMID: 30362089 Free PMC article. Review. - DNA Methylation Patterns According to Fatty Liver Index and Longitudinal Changes from the Korean Genome and Epidemiology Study (KoGES).
Ko YK, Kim H, Lee Y, Lee YS, Gim JA. Ko YK, et al. Curr Issues Mol Biol. 2022 Feb 27;44(3):1149-1168. doi: 10.3390/cimb44030075. Curr Issues Mol Biol. 2022. PMID: 35723298 Free PMC article. - The epigenetic landscape of alcoholism.
Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC. Krishnan HR, et al. Int Rev Neurobiol. 2014;115:75-116. doi: 10.1016/B978-0-12-801311-3.00003-2. Int Rev Neurobiol. 2014. PMID: 25131543 Free PMC article. Review. - Acetylation and deacetylation in cancer stem-like cells.
Liu N, Li S, Wu N, Cho KS. Liu N, et al. Oncotarget. 2017 Jul 11;8(51):89315-89325. doi: 10.18632/oncotarget.19167. eCollection 2017 Oct 24. Oncotarget. 2017. PMID: 29179522 Free PMC article. Review.
References
- Alison MR, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: The good, the bad and the ugly. Journal of Pathology. 2009;217(2):282–298. - PubMed
- Bardag-Gorce F, French BA, Nan L, et al. CYP2E1 induced by ethanol causes oxidative stress, proteasome inhibition and cytokeratin aggresome (Mallory body-like) formation. Experimental and Molecular Pathology. 2006;81(3):191–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical