Toll-like receptors in antiviral innate immunity - PubMed (original) (raw)
Review
Toll-like receptors in antiviral innate immunity
Sandra N Lester et al. J Mol Biol. 2014.
Abstract
Toll-like receptors (TLRs) are fundamental sensor molecules of the host innate immune system, which detect conserved molecular signatures of a wide range of microbial pathogens and initiate innate immune responses via distinct signaling pathways. Various TLRs are implicated in the early interplay of host cells with invading viruses, which regulates viral replication and/or host responses, ultimately impacting on viral pathogenesis. To survive the host innate defense mechanisms, many viruses have developed strategies to evade or counteract signaling through the TLR pathways, creating an advantageous environment for their propagation. Here we review the current knowledge of the roles TLRs play in antiviral innate immune responses, discuss examples of TLR-mediated viral recognition, and describe strategies used by viruses to antagonize the host antiviral innate immune responses.
Keywords: cytokine; interferon; interferon regulatory factor; nuclear factor-kappa B; virus.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
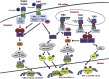
Graphical abstract
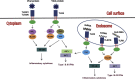
Fig. 1
Recognition of viral PAMPs such as viral proteins, dsRNA, ssRNA, and CpG DNA, initiates an antiviral innate immune response mediated by TLRs. TLR2 and TLR4 are present on the cell surface and recognize viral proteins. TLR3, TLR7, TLR8, and TLR9 are intracellular viral nucleic-acid-sensing TLRs that are localized in endosomes. Viral dsRNA, ssRNA, and unmethylated CpG DNA are recognized by TLR3, TLR7/TLR8, and TLR9, respectively. Upon ligand recognition, TLR2 along with TLR6 or TLR1 and TLR4 recruits an additional adaptor protein, MAL, to link the TIR domain to MyD88. All TLRs except TLR3 recruit MyD88. TLR4 also recruits the adapter protein TRIF, as does TLR3. To activate the TRIF-dependent pathway, TLR4 requires the bridging adaptor TRAM and its trafficking into endosomes. The MyD88-dependent and TRIF-dependent signaling complexes through a cascade of signaling events leading to the activation of several transcription factors including NF-κB, IRF3, and IRF7. NF-κB transcriptionally regulates the expression of inflammatory cytokines and chemokines while IRF3 and IRF7 control the transcription of type I and type III IFN genes. Whereas TLR2 signaling only results in NF-κB activation in most cell types, TLR2 can traffic to endosomes in inflammatory monocytes upon engagement of specific viral ligands such as vaccinia virus or MCMV where it results in IRF3/IRF7 activation and type I IFN induction (not depicted).
Similar articles
- Recognition of herpes simplex viruses: toll-like receptors and beyond.
Ma Y, He B. Ma Y, et al. J Mol Biol. 2014 Mar 20;426(6):1133-47. doi: 10.1016/j.jmb.2013.11.012. Epub 2013 Nov 19. J Mol Biol. 2014. PMID: 24262390 Free PMC article. Review. - Toll-like receptors, innate immunity and HSV pathogenesis.
Herbst-Kralovetz M, Pyles R. Herbst-Kralovetz M, et al. Herpes. 2006 Aug;13(2):37-41. Herpes. 2006. PMID: 16895653 Review. - Toll-like receptors as novel therapeutic targets for herpes simplex virus infection.
Jahanban-Esfahlan R, Seidi K, Majidinia M, Karimian A, Yousefi B, Nabavi SM, Astani A, Berindan-Neagoe I, Gulei D, Fallarino F, Gargaro M, Manni G, Pirro M, Xu S, Sadeghi M, Nabavi SF, Shirooie S. Jahanban-Esfahlan R, et al. Rev Med Virol. 2019 Jul;29(4):e2048. doi: 10.1002/rmv.2048. Epub 2019 Jul 2. Rev Med Virol. 2019. PMID: 31265195 Review. - Toll-like Receptors in Viral Encephalitis.
Gern OL, Mulenge F, Pavlou A, Ghita L, Steffen I, Stangel M, Kalinke U. Gern OL, et al. Viruses. 2021 Oct 14;13(10):2065. doi: 10.3390/v13102065. Viruses. 2021. PMID: 34696494 Free PMC article. Review. - Toll-like receptor-mediated recognition of herpes simplex virus.
Martinez-Martin N, Viejo-Borbolla A. Martinez-Martin N, et al. Front Biosci (Schol Ed). 2010 Jan 1;2(2):718-29. doi: 10.2741/s96. Front Biosci (Schol Ed). 2010. PMID: 20036979 Review.
Cited by
- Molecular characterization and functional analysis of DIGIRR from golden pompano (Trachinotus ovatus).
Xie Y, Gao S, Cao Y, Ji Y, Zhang Q, Wei Y, Qi Z. Xie Y, et al. Front Immunol. 2022 Aug 24;13:974310. doi: 10.3389/fimmu.2022.974310. eCollection 2022. Front Immunol. 2022. PMID: 36091048 Free PMC article. - Exploration of surface glycoprotein to design multi-epitope vaccine for the prevention of Covid-19.
Oladipo EK, Ajayi AF, Ariyo OE, Onile SO, Jimah EM, Ezediuno LO, Adebayo OI, Adebayo ET, Odeyemi AN, Oyeleke MO, Oyewole MP, Oguntomi AS, Akindiya OE, Olamoyegun BO, Aremu VO, Arowosaye AO, Aboderin DO, Bello HB, Senbadejo TY, Awoyelu EH, Oladipo AA, Oladipo BB, Ajayi LO, Majolagbe ON, Oyawoye OM, Oloke JK. Oladipo EK, et al. Inform Med Unlocked. 2020;21:100438. doi: 10.1016/j.imu.2020.100438. Epub 2020 Oct 4. Inform Med Unlocked. 2020. PMID: 33043110 Free PMC article. - Cathelicidins Modulate TLR-Activation and Inflammation.
Scheenstra MR, van Harten RM, Veldhuizen EJA, Haagsman HP, Coorens M. Scheenstra MR, et al. Front Immunol. 2020 Jun 9;11:1137. doi: 10.3389/fimmu.2020.01137. eCollection 2020. Front Immunol. 2020. PMID: 32582207 Free PMC article. Review. - Acute Kidney Injury: Definition, Management, and Promising Therapeutic Target.
Almazmomi MA, Esmat A, Naeem A. Almazmomi MA, et al. Cureus. 2023 Dec 28;15(12):e51228. doi: 10.7759/cureus.51228. eCollection 2023 Dec. Cureus. 2023. PMID: 38283512 Free PMC article. Review. - Gastrointestinal Motility and Improvement Efficacy of Shenhuang Plaster Application on Shenque: Identification, Evaluation, and Mechanism.
Shi Y, Xu J, Ding B, Chen G, Jin L, Ke L, Xu X, Wang J, Sun Q, Xu X. Shi Y, et al. J Immunol Res. 2020 Jul 11;2020:2383970. doi: 10.1155/2020/2383970. eCollection 2020. J Immunol Res. 2020. PMID: 32733972 Free PMC article.
References
- Kawai T., Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. - PubMed
- Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. - PubMed
- Medzhitov R., Janeway C.A. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. - PubMed
- Palsson-McDermott E.M., O'Neill L.A. Building an immune system from nine domains. Biochem Soc Trans. 2007;35:1437–1444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI078906/AI/NIAID NIH HHS/United States
- R56 AI069285/AI/NIAID NIH HHS/United States
- R01 AI069285/AI/NIAID NIH HHS/United States
- T32 AI078906/AI/NIAID NIH HHS/United States
- R21 AI101526/AI/NIAID NIH HHS/United States
- AI101526/AI/NIAID NIH HHS/United States
- AI069285/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical