Evolution at two levels of gene expression in yeast - PubMed (original) (raw)
Evolution at two levels of gene expression in yeast
Carlo G Artieri et al. Genome Res. 2014 Mar.
Abstract
Despite the greater functional importance of protein levels, our knowledge of gene expression evolution is based almost entirely on studies of mRNA levels. In contrast, our understanding of how translational regulation evolves has lagged far behind. Here we have applied ribosome profiling--which measures both global mRNA levels and their translation rates--to two species of Saccharomyces yeast and their interspecific hybrid in order to assess the relative contributions of changes in mRNA abundance and translation to regulatory evolution. We report that both cis- and trans-acting regulatory divergence in translation are abundant, affecting at least 35% of genes. The majority of translational divergence acts to buffer changes in mRNA abundance, suggesting a widespread role for stabilizing selection acting across regulatory levels. Nevertheless, we observe evidence of lineage-specific selection acting on several yeast functional modules, including instances of reinforcing selection acting at both levels of regulation. Finally, we also uncover multiple instances of stop-codon readthrough that are conserved between species. Our analysis reveals the underappreciated complexity of post-transcriptional regulatory divergence and indicates that partitioning the search for the locus of selection into the binary categories of "coding" versus "regulatory" may overlook a significant source of selection, acting at multiple regulatory levels along the path from genotype to phenotype.
Figures
Figure 1.
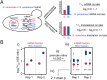
(A) Identifying _cis-_regulatory divergence at two levels. In the example, the S. paradoxus allele (blue) is transcribed at a higher level than that of S. cerevisiae (red), as represented by the larger number of wavy lines. However, the S. cerevisiae allele has higher translational efficiency, as represented by the larger number of ribosomes per transcript (pairs of gray circles). The S. paradoxus mRNA cis bias manifests as a negative log2(Sc/Sp) ratio in the mRNA fraction. If translational efficiency was unchanged between alleles, the more abundant allele, in this case S. paradoxus, would produce more footprints in the Ribo fraction. Therefore the translational cis ratio is obtained by dividing the Sc/Sp Ribo fraction ratio by the mRNA fraction ratio (which is equivalent to a subtraction in log2). The net log2(Sc/Sp) translational cis ratio is positive, indicating cis bias favoring S. cerevisiae translation. (B) Detection of significant translational divergence is based on rejecting the null hypothesis that the observed allelic ratios are not significantly different from one another (see A). The observed Sc/Sp ratios (red circles, mRNA fraction; blue circles, Ribo fraction) (i) were obtained directly from the replicates of the two fractions. (ii) These were permuted by resampling the base-level coverage of each allele with replacement 10,000 times, generating a distribution of Sc/Sp ratios that captures the interallelic variability in base composition, length, and read coverage. (iii) The distributions of permuted ratios (boxplots) were then each reciprocally compared with the corresponding observed ratio (e.g., the permuted distribution of Sc/Sp Ribo ratios [blue boxplots] was compared with the observed mRNA Sc/Sp ratio [red circles] and vice versa) for which a two-tailed _P_-value was calculated. If all comparisons agreed in the parental direction of allelic bias, then (iv) the least significant _P_-value (indicated by the red asterisk) was used as the representative for the comparison. See Supplemental Material for application of the test to the mRNA level and trans comparisons.
Figure 2.
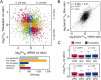
(A) The relationship between _cis_-regulatory divergence at the mRNA abundance and translational levels (all plotted Sc/Sp ratios are the mean of the two biological replicates). Divergence was detected only at the mRNA level for a large fraction of genes (orange circles), though greater than one-tenth of orthologs were significantly diverged only in translation (blue circles). Among orthologs diverged at both levels, we observed a significant excess opposing (red triangles) as compared with reinforcing changes (green squares). The number of orthologs in each class is indicated in the barplot. (S. cer) S. cerevisiae; (S. par) S. paradoxus. (B) Opposing divergence across regulatory levels. The red line indicates the best fit of a linear regression, with equation, p, and _r_2 values indicated above. The slope is significantly lower than one (95% confidence interval ±0.033), indicating that Sc/Sp mRNA ratio estimates tend to overestimate the degree of difference by ∼15% relative to that of the Ribo fraction. (C) Orthologs whose promoters contain either TATA boxes (TATA) or occupied proximal nucleosome regions (OPN) (Tirosh and Barkai 2008) show more divergence in cis only at the mRNA level when compared with non-TATA promoters (Non) or depleted proximal nucleosome regions (DPNs), respectively. Kruskal–Wallis test _P_-values are indicated above each fraction.
Figure 3.
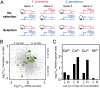
(A) Detecting selection from patterns of ASE in hybrids. The example above shows ASE levels (indicated by the wavy lines) for four genes belonging to a particular functional category. Black “X”s indicate down-regulating _cis_-regulatory differences between the parental alleles. For a given group of functionally related genes evolving neutrally, no bias is expected with respect to the directionality of ASE in hybrids (No selection). However, biased directionality, as in the case in which all down-regulating mutations occurred along the S. cerevisiae lineage (Selection), indicates a history of lineage-specific selection acting on _cis_-regulation. (B) Reinforcing lineage-specific bias on orthologs involved in divalent cation and heavy metal resistance. (Green triangles) Orthologs within this functional category with reinforcing directionality of bias at both regulatory levels. Significantly more (17) orthologs are reinforcing along the S. cerevisiae lineage as compared with that of S. paradoxus (five). All orthologs are indicated as gray circles. (C) S. cerevisiae strain S288c is more resistant to heavy metals than S. paradoxus strain CBS432. Shown are the log2-transformed relative growth rates (S. cerevisiae/S. paradoxus) for the four heavy metals at two concentrations (L, low; H, high) measured by Warringer et al. (2011). S. cerevisiae outperforms S. paradoxus under all conditions, although in the presence of nickel, the difference is negligible.
Figure 4.
Evidence of stop-codon readthrough leading to C-terminal peptide extension. The translation initiation codons are indicated by the _right_-facing arrow, the annotated ORF by the thick black lines, and the canonical stop codon by the black triangles. The candidate C-terminal peptide extension is indicated by the gray line terminated by in-frame stop codons in the 3′ UTR (gray triangles above the line for S. cerevisiae, and below for S. paradoxus). Dark shades (red, S. cerevisiae; blue, S. paradoxus) indicate nucleotide-level coverage of mRNA fraction reads, and light shades indicate Ribo fraction reads. (A) Example of conserved C-terminal peptide extension of the translation initiation factor eIF1A (TIF11). The putative 21-amino-acid extension is conserved and well covered by reads in the Ribo fraction of both species. (B) Example of a _S. paradoxus_–specific C-terminal extension in MRPS16, a subunit of the mitochondrial ribosome. mRNA fraction reads indicate that the 3′ UTR is expressed in both species; however, translation is only detected in the 17-amino-acid extension of S. paradoxus, and not the potential 21-amino-acid extension of S. cerevisiae. Interestingly, coverage of the C-terminal extension in S. paradoxus is comparable to that of the CDS, suggesting that readthrough of this gene may be frequent.
Similar articles
- Ribosome profiling reveals post-transcriptional buffering of divergent gene expression in yeast.
McManus CJ, May GE, Spealman P, Shteyman A. McManus CJ, et al. Genome Res. 2014 Mar;24(3):422-30. doi: 10.1101/gr.164996.113. Epub 2013 Dec 6. Genome Res. 2014. PMID: 24318730 Free PMC article. - Genetic influences on translation in yeast.
Albert FW, Muzzey D, Weissman JS, Kruglyak L. Albert FW, et al. PLoS Genet. 2014 Oct 23;10(10):e1004692. doi: 10.1371/journal.pgen.1004692. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25340754 Free PMC article. - Transcriptome-wide investigation of stop codon readthrough in Saccharomyces cerevisiae.
Mangkalaphiban K, He F, Ganesan R, Wu C, Baker R, Jacobson A. Mangkalaphiban K, et al. PLoS Genet. 2021 Apr 20;17(4):e1009538. doi: 10.1371/journal.pgen.1009538. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33878104 Free PMC article. - Selection for more of the same product as a force to enhance concerted evolution of duplicated genes.
Sugino RP, Innan H. Sugino RP, et al. Trends Genet. 2006 Dec;22(12):642-4. doi: 10.1016/j.tig.2006.09.014. Epub 2006 Oct 11. Trends Genet. 2006. PMID: 17045359 Review. - The transcriptional apparatus required for mRNA encoding genes in the yeast Saccharomyces cerevisiae emerges from a jigsaw puzzle of transcription factors.
Künzler M, Springer C, Braus GH. Künzler M, et al. FEMS Microbiol Rev. 1996 Dec;19(2):117-36. doi: 10.1111/j.1574-6976.1996.tb00256.x. FEMS Microbiol Rev. 1996. PMID: 8988567 Review.
Cited by
- Coordinated Evolution of Transcriptional and Post-Transcriptional Regulation for Mitochondrial Functions in Yeast Strains.
Sun X, Wang Z, Guo X, Li H, Gu Z. Sun X, et al. PLoS One. 2016 Apr 14;11(4):e0153523. doi: 10.1371/journal.pone.0153523. eCollection 2016. PLoS One. 2016. PMID: 27077367 Free PMC article. - Unbiased Quantitative Models of Protein Translation Derived from Ribosome Profiling Data.
Gritsenko AA, Hulsman M, Reinders MJ, de Ridder D. Gritsenko AA, et al. PLoS Comput Biol. 2015 Aug 14;11(8):e1004336. doi: 10.1371/journal.pcbi.1004336. eCollection 2015 Aug. PLoS Comput Biol. 2015. PMID: 26275099 Free PMC article. - Genome and transcriptome evolve separately in recently hybridized Trichosporon fungi.
Sriswasdi S, Takashima M, Manabe RI, Ohkuma M, Iwasaki W. Sriswasdi S, et al. Commun Biol. 2019 Jul 19;2:263. doi: 10.1038/s42003-019-0515-2. eCollection 2019. Commun Biol. 2019. PMID: 31341962 Free PMC article. - Improving Estimates of Compensatory cis-trans Regulatory Divergence.
Fraser HB. Fraser HB. Trends Genet. 2019 Jan;35(1):3-5. doi: 10.1016/j.tig.2018.09.003. Epub 2018 Sep 27. Trends Genet. 2019. PMID: 30270122 Free PMC article. Review. - The use of duplex-specific nuclease in ribosome profiling and a user-friendly software package for Ribo-seq data analysis.
Chung BY, Hardcastle TJ, Jones JD, Irigoyen N, Firth AE, Baulcombe DC, Brierley I. Chung BY, et al. RNA. 2015 Oct;21(10):1731-45. doi: 10.1261/rna.052548.115. Epub 2015 Aug 18. RNA. 2015. PMID: 26286745 Free PMC article.
References
- Barrett RD, Hoekstra HE 2011. Molecular spandrels: Tests of adaptation at the genetic level. Nat Rev Genet 12: 767–780 - PubMed
- Benjamini Y, Hochberg Y 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57: 289–300
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases