Review series: From uncertain beginnings: initiation mechanisms of clathrin-mediated endocytosis - PubMed (original) (raw)
Review
Review series: From uncertain beginnings: initiation mechanisms of clathrin-mediated endocytosis
Camilla Godlee et al. J Cell Biol. 2013.
Abstract
Clathrin-mediated endocytosis is a central and well-studied trafficking process in eukaryotic cells. How this process is initiated is likely to be a critical point in regulating endocytic activity spatially and temporally, but the underlying mechanisms are poorly understood. During the early stages of endocytosis three components-adaptor and accessory proteins, cargo, and lipids-come together at the plasma membrane to begin the formation of clathrin-coated vesicles. Although different models have been proposed, there is still no clear picture of how these three components cooperate to initiate endocytosis, which may indicate that there is some flexibility underlying this important event.
Figures
Figure 1.
Examples of regulation of the initiation of endocytosis. (A) In yeast Saccharomyces cerevisiae, clathrin-mediated endocytosis is polarized to the growing bud. (B) After signal propagation at the synapse, exocytosis of the neurotransmitter is accompanied by a compensatory increase in clathrin-mediated endocytosis to maintain synaptic plasma membrane area and recycle synaptic vesicle components. (C) Many viruses enter the host cell by clathrin-mediated endocytosis. The virus particle may exploit the cell’s regulation of endocytosis in order to induce its own uptake.
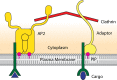
Figure 2.
The key players involved in the initiation of endocytosis. Adaptor proteins, cargo, lipids, and clathrin accumulate at the plasma membrane to make up the nascent endocytic site. Adaptor proteins, including the AP2 complex, bind to the inner leaflet of the plasma membrane. The adaptors interact with the plasma membrane via lipid-binding domains, many of which bind specifically to PIP2. The tails of adaptor proteins bind and recruit clathrin triskelions to the plasma membrane where they polymerize to form the clathrin coat. Cargo—consisting of transmembrane receptors and extracellular soluble ligands—is taken up by clathrin-coated vesicles. Adaptors bind to signaling motifs on the intracellular side of the receptors.
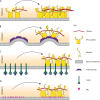
Figure 3.
Possible mechanisms for the initiation of endocytosis. (A) Stochastic interactions of adaptors and lipids at the plasma membrane can form a nucleus that recruits further endocytic components. One such assembly that has been proposed is two AP2 complexes along with a clathrin triskelion (Cocucci et al., 2012). (B) Membrane bending may be required or aid in the initiation of endocytosis. Protein domains such as the F-BAR domain have been suggested to induce membrane bending as a mechanism for initiating endocytic site formation (Henne et al., 2010). (C) Clustering of cargo molecules has been shown to be able to induce the assembly of endocytic components de novo (Liu et al., 2010). (D) Specific lipids forming a lipid gradient or at a membrane domain could increase the recruitment of adaptors to the plasma membrane, leading to increased initiation of endocytosis.
Similar articles
- Endocytic accessory factors and regulation of clathrin-mediated endocytosis.
Merrifield CJ, Kaksonen M. Merrifield CJ, et al. Cold Spring Harb Perspect Biol. 2014 Oct 3;6(11):a016733. doi: 10.1101/cshperspect.a016733. Cold Spring Harb Perspect Biol. 2014. PMID: 25280766 Free PMC article. Review. - Mechanisms of clathrin-mediated endocytosis.
Kaksonen M, Roux A. Kaksonen M, et al. Nat Rev Mol Cell Biol. 2018 May;19(5):313-326. doi: 10.1038/nrm.2017.132. Epub 2018 Feb 7. Nat Rev Mol Cell Biol. 2018. PMID: 29410531 Review. - Budding and braking news about clathrin-mediated endocytosis.
Baisa GA, Mayers JR, Bednarek SY. Baisa GA, et al. Curr Opin Plant Biol. 2013 Dec;16(6):718-25. doi: 10.1016/j.pbi.2013.09.005. Epub 2013 Oct 15. Curr Opin Plant Biol. 2013. PMID: 24139529 Review. - Molecular mechanism and physiological functions of clathrin-mediated endocytosis.
McMahon HT, Boucrot E. McMahon HT, et al. Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):517-33. doi: 10.1038/nrm3151. Nat Rev Mol Cell Biol. 2011. PMID: 21779028 Review. - Regulation of Clathrin-Mediated Endocytosis.
Mettlen M, Chen PH, Srinivasan S, Danuser G, Schmid SL. Mettlen M, et al. Annu Rev Biochem. 2018 Jun 20;87:871-896. doi: 10.1146/annurev-biochem-062917-012644. Epub 2018 Apr 16. Annu Rev Biochem. 2018. PMID: 29661000 Free PMC article. Review.
Cited by
- Plasma membrane proteomic analysis of human Gastric Cancer tissues: revealing flotillin 1 as a marker for Gastric Cancer.
Gao W, Xu J, Wang F, Zhang L, Peng R, Shu Y, Wu J, Tang Q, Zhu Y. Gao W, et al. BMC Cancer. 2015 May 7;15:367. doi: 10.1186/s12885-015-1343-5. BMC Cancer. 2015. PMID: 25948494 Free PMC article. - The biogenesis of potassium transporters: implications of disease-associated mutations.
Kok M, Brodsky JL. Kok M, et al. Crit Rev Biochem Mol Biol. 2024 Jun-Aug;59(3-4):154-198. doi: 10.1080/10409238.2024.2369986. Epub 2024 Jul 1. Crit Rev Biochem Mol Biol. 2024. PMID: 38946646 Review. - Structural organization and dynamics of FCHo2 docking on membranes.
El Alaoui F, Casuso I, Sanchez-Fuentes D, Arpin-Andre C, Rathar R, Baecker V, Castro A, Lorca T, Viaud J, Vassilopoulos S, Carretero-Genevrier A, Picas L. El Alaoui F, et al. Elife. 2022 Jan 19;11:e73156. doi: 10.7554/eLife.73156. Elife. 2022. PMID: 35044298 Free PMC article. - Sorting Nexins in Protein Homeostasis.
Hanley SE, Cooper KF. Hanley SE, et al. Cells. 2020 Dec 24;10(1):17. doi: 10.3390/cells10010017. Cells. 2020. PMID: 33374212 Free PMC article. Review. - Crosslinked-hybrid nanoparticle embedded in thermogel for sustained co-delivery to inner ear.
Thakur NS, Rus I, Herbert A, Zallocchi M, Chakrabarty B, Joshi AD, Lomeo J, Agrahari V. Thakur NS, et al. J Nanobiotechnology. 2024 Aug 13;22(1):482. doi: 10.1186/s12951-024-02686-z. J Nanobiotechnology. 2024. PMID: 39135039 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources