The effects of estrogen in ischemic stroke - PubMed (original) (raw)
Review
The effects of estrogen in ischemic stroke
Edward C Koellhoffer et al. Transl Stroke Res. 2013 Aug.
Abstract
Stroke is a leading cause of death and the most common cause of long-term disability in the USA. Women have a lower incidence of stroke compared with men throughout most of the lifespan which has been ascribed to protective effects of gonadal steroids, most notably estrogen. Due to the lower stroke incidence observed in pre-menopausal women and robust preclinical evidence of neuroprotective and anti-inflammatory properties of estrogen, researchers have focused on the potential benefits of hormones to reduce ischemic brain injury. However, as women age, they are disproportionately affected by stroke, coincident with the loss of estrogen with menopause. The risk of stroke in elderly women exceeds that of men and it is clear that in some settings estrogen can have pro-inflammatory effects. This review will focus on estrogen and inflammation and its interaction with aging.
Figures
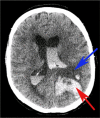
Fig. 1
Case of intraventricular and intracerebral hemorrhage following treatment with tPA 70-year-old man with left middle cerebral artery stroke who was administered tPA and subsequently worsened. Repeat CT scan showed intraventricular and intracerebral hemorrhage as well as midline shift. Blue arrow indicates ischemic infarct and red arrow indicates hemorrhage
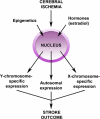
Fig. 2
Influence of epigenetics and hormones on genetic expression and stroke outcome
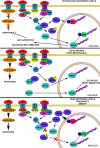
Fig. 3
Sexual dimorphism in cell death following cerebral ischemia Caspase-dependent death predominates in females, where the influx of Ca2+ induces formation of the MAC which permeabilizes the mitochondrial membrane. Cytochrome c, Smac/DIABLO, and X-linked inhibitor of apoptosis protein (XIAP) are released into the cytosol. Cytochrome c and caspase-9 facilitate formation of the apoptosome which in turn activates caspase-3. Caspase-3 cleaves the inhibitor of caspase-activated DNase to give caspase-activated DNase (CAD) which subsequently cleaves DNA and results in apoptosis. Caspase-independent cell death predominates in males, in which ischemia results in an increase in reactive oxygen species (ROS) which combine with nitric oxide (NO) to form peroxynitrite (_ONOO_–). ONOO– damages DNA via oxygenation and nitration which activates polymerase (ADP-ribose), polymerase-1 (_PARP_-1), and formation of poly(ADP-ribose) (PAR) polymers. Permeabilization of the mitochondrial membrane enables apoptosis-inducing factor (AIF) to translocate from the intermembrane space of the mitochondrion to the nucleus where it causes specific DNA fragmentation patterns and chromatin condensation, ultimately resulting in apoptosis
Fig. 4
Hypothesized interaction between estradiol, TNF, and NFκB signaling At physiological levels of estradiol (E2), tumor necrosis factor (TNF) preferentially binds to TNF receptor 2 (TNFR2), denoted R2, leading to activation of nuclear factor-κ B (NFκB) which translocates to the nucleus. Balance between estrogen receptor-α (ERα) transcription of inhibitor of κ B-α (IκBα) and estrogen receptor-β (ERβ) inhibition of NFκB binding is present, resulting in balanced inflammation and net anti-apoptotic signaling. With physiological levels of E2, TNF levels are lower and there is reduced stimulation of TNF receptor 1 (TNFR1), denoted R1, and subsequent caspase signaling. In postmenopausal women, E2 levels decline and there is a lack of ERα-induced IκBα expression and ERβ-mediated inhibition of NFκB. Higher circulating TNF levels stimulate both TNFR2 and TNFR1, leading to relatively uninhibited inflammation and induction of caspase-dependent apoptosis. With high levels of E2 as seen in pregnancy and obesity, there is increased expression of IκBα which inhibits NFκB. The NFκB that translocates to the nucleus is inhibited by ERβ binding, inhibiting expression of anti-apoptotic genes by NFκB. Lack of inhibition of TNFR1 signaling results in caspase-dependent apoptosis. See reviews [120, 144]
Similar articles
- Sex differences in ischaemic stroke: potential cellular mechanisms.
Chauhan A, Moser H, McCullough LD. Chauhan A, et al. Clin Sci (Lond). 2017 Apr 1;131(7):533-552. doi: 10.1042/CS20160841. Clin Sci (Lond). 2017. PMID: 28302915 Review. - Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke.
Ritzel RM, Capozzi LA, McCullough LD. Ritzel RM, et al. Horm Behav. 2013 Feb;63(2):238-53. doi: 10.1016/j.yhbeh.2012.04.007. Epub 2012 Apr 24. Horm Behav. 2013. PMID: 22561337 Free PMC article. Review. - Experimental pediatric arterial ischemic stroke model reveals sex-specific estrogen signaling.
Herson PS, Bombardier CG, Parker SM, Shimizu T, Klawitter J, Klawitter J, Quillinan N, Exo JL, Goldenberg NA, Traystman RJ. Herson PS, et al. Stroke. 2013 Mar;44(3):759-63. doi: 10.1161/STROKEAHA.112.675124. Epub 2013 Jan 24. Stroke. 2013. PMID: 23349190 Free PMC article. - Stroke in the female: role of biological sex and estrogen.
Murphy SJ, McCullough LD, Smith JM. Murphy SJ, et al. ILAR J. 2004;45(2):147-59. doi: 10.1093/ilar.45.2.147. ILAR J. 2004. PMID: 15111734 Review. - Sexual dimorphism in ischemic stroke: lessons from the laboratory.
Manwani B, McCullough LD. Manwani B, et al. Womens Health (Lond). 2011 May;7(3):319-39. doi: 10.2217/whe.11.22. Womens Health (Lond). 2011. PMID: 21612353 Free PMC article. Review.
Cited by
- Vagus nerve stimulation is a potential treatment for ischemic stroke.
Liu YL, Wang SR, Ma JX, Yu LH, Jia GW. Liu YL, et al. Neural Regen Res. 2023 Apr;18(4):825-831. doi: 10.4103/1673-5374.350698. Neural Regen Res. 2023. PMID: 36204850 Free PMC article. - Neuroprotective effects of psilocybin in a rat model of stroke.
Yu SJ, Wu KJ, Wang YS, Bae E, Chianelli F, Bambakidis N, Wang Y. Yu SJ, et al. BMC Neurosci. 2024 Oct 8;25(1):49. doi: 10.1186/s12868-024-00903-x. BMC Neurosci. 2024. PMID: 39379834 Free PMC article. - cGMP-dependent protein kinase I in vascular smooth muscle cells improves ischemic stroke outcome in mice.
Shvedova M, Litvak MM, Roberts JD Jr, Fukumura D, Suzuki T, Şencan İ, Li G, Reventun P, Buys ES, Kim HH, Sakadžić S, Ayata C, Huang PL, Feil R, Atochin DN. Shvedova M, et al. J Cereb Blood Flow Metab. 2019 Dec;39(12):2379-2391. doi: 10.1177/0271678X19870583. Epub 2019 Aug 18. J Cereb Blood Flow Metab. 2019. PMID: 31423931 Free PMC article. - X, but not Y, Chromosomal Complement Contributes to Stroke Sensitivity in Aged Animals.
Qi S, Ngwa C, Al Mamun A, Romana S, Wu T, Marrelli SP, Arnold AP, McCullough LD, Liu F. Qi S, et al. Transl Stroke Res. 2023 Oct;14(5):776-789. doi: 10.1007/s12975-022-01070-z. Epub 2022 Jul 29. Transl Stroke Res. 2023. PMID: 35906327 Free PMC article. - Hormones and Sex-Specific Medicine in Human Physiopathology.
Tokatli MR, Sisti LG, Marziali E, Nachira L, Rossi MF, Amantea C, Moscato U, Malorni W. Tokatli MR, et al. Biomolecules. 2022 Mar 7;12(3):413. doi: 10.3390/biom12030413. Biomolecules. 2022. PMID: 35327605 Free PMC article. Review.
References
- Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28(3):491–9. - PubMed
- Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24(4):458–66. doi:10.1097/00004647-200404000-00011. - PubMed
- Yu SW, Wang H, Dawson TM, Dawson VL. Poly(ADP-ribose) polymerase-1 and apoptosis inducing factor in neurotoxicity. Neurobiol Dis. 2003;14(3):303–17. - PubMed
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–7. doi:10.1056/NEJM199512143332401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical