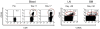Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells - PubMed (original) (raw)
Review
Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells
Michael C Jensen et al. Immunol Rev. 2014 Jan.
Erratum in
- Immunol Rev. 2014 Mar;258(1):259
Abstract
A major advance in adoptive T-cell therapy (ACT) is the ability to efficiently endow patient's T cells with reactivity for tumor antigens through the stable or regulated introduction of genes that encode high affinity tumor-targeting T-cell receptors (TCRs) or synthetic chimeric antigen receptors (CARs). Case reports and small series of patients treated with TCR- or CAR-modified T cells have shown durable responses in a subset of patients, particularly with B-cell malignancies treated with T cells modified to express a CAR that targets the CD19 molecule. However, many patients do not respond to therapy and serious on and off-target toxicities have been observed with TCR- and CAR-modified T cells. Thus, challenges remain to make ACT with gene-modified T cells a reproducibly effective and safe therapy and to expand the breadth of patients that can be treated to include those with common epithelial malignancies. This review discusses research topics in our laboratories that focus on the design and implementation of ACT with CAR-modified T cells. These include cell intrinsic properties of distinct T-cell subsets that may facilitate preparing therapeutic T-cell products of defined composition for reproducible efficacy and safety, the design of tumor targeting receptors that optimize signaling of T-cell effector functions and facilitate tracking of migration of CAR-modified T cells in vivo, and novel CAR designs that have alternative ligand binding domains or confer regulated function and/or survival of transduced T cells.
Keywords: T cells; T-cell receptors; cancer; chimeric antigen receptors; gene therapy; immunotherapies.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Fig. 1. Linear differentiation of T-cell subsets
The phenotype of naive, memory, and effector subsets is shown and the linear pathway of differentiation from a naive T cell is based on recent data from fate mapping studies in murine models (40,41).
Fig. 2. High-level persistence and migration of adoptively transferred T cells derived from a single central memory T (Tcm) cell
CD8+ Tcm cells were sort-purified based on expression of CD62L and CD9,5 and single T cells specific for cytomegalovirus were derived and expanded in limiting dilution cultures and retrovirally marked with a truncated CD19 molecule to facilitate detection in vivo. The expanded, gene marked T cells were adoptively transferred to the animal without preceding lymphodepletion or the administration of cytokines post infusion. The frequency of gene marked (CD19+) CD8+ T cells in the CD8+ T cell subset in blood, lymph nodes (LN), and bone marrow (BM) is shown.
Fig. 3. Elements of synthetic chimeric antigen receptors
Schematic of ligand binding, non-signaling, and signaling elements of a CAR that can be altered to optimize tumor cell recognition and signaling of T-cell function.
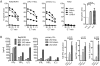
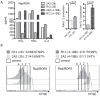
Fig. 4. Effect of CAR ScFv affinity on T-cell effector function and proliferation
(A) Multiplex cytokine analysis after a 24-hour stimulation of 5×104 CAR-modified T cells expressing a ROR-1 specific CAR constructed with the 2A2 scFv or with the higher affinity R12 scFv with primary Raji lymphoma cells that express ROR1. Data are shown for CAR designs that include either the CD28 or 4-1BB costimulatory domains. The right panels show the fold increase in IL-2 production. (B) Proliferation of carboxyfluorescein-labeled CD8+ T cells modified with the 2A2 ROR1 and high affinity R12 ROR1 CARs 72 h after stimulation with primary CLL cells. Numbers above each histogram indicate the number of cell divisions, and the fraction of T-cells in each gate that underwent ≥3/2/1 cell divisions is provided above each plot.
Fig. 5. Effect of non-signaling extracellular spacer domain length on recognition of ROR1+ tumor cells with a ROR1 CAR specific for a membrane distal target epitope
(A) Cytolytic activity of T cells expressing the 2A2 ROR1-CAR with a long, intermediate, or short extracellular spacer domain against ROR1+ tumor cells and control K562 cells. Control T cells are modified with the EGFRt marker gene only. Cytotoxicity data from 4 independent experiments (E:T = 30:1) were normalized (cytolytic activity by ROR1-CAR 2A2 = 1) and analyzed by Student’s t-test (bar diagram). (C) Multiplex cytokine analysis after a 24-h stimulation of 5×104 CAR T-cells with Raji/ROR1 cells and primary CLL cells.
Fig. 6. Split receptor systems for conditional tumor recognition
(A) Conceptual schematic of a split receptor that functions as an ‘AND’ logic gate. Tumor specificity is achieved when two cell surface antigens are uniquely co-expressed on tumor cells. The split receptor AND logic system targets the combination of the two antigens utilizing both an attenuated activation receptor CAR housing a CD3-ζ domain and a second receptor housing costimulatory intracellular signaling domains. Only the combination of the two receptors engaging antigen results in the activation of the T cell. (B) The concept of a system that is permissive of T-cell activation when antigen is present on tumor cells and a second antigen is absent, wherein the second antigen is present on normal cells. In this split receptor system the chimeric receptor for the inhibiting second receptor acts as in a dominant negative manner to prevent T-cell activation when both antigens are encountered on the same cell.
Similar articles
- At the Bench: Chimeric antigen receptor (CAR) T cell therapy for the treatment of B cell malignancies.
Daniyan AF, Brentjens RJ. Daniyan AF, et al. J Leukoc Biol. 2016 Dec;100(6):1255-1264. doi: 10.1189/jlb.5BT1215-556RR. Epub 2016 Oct 27. J Leukoc Biol. 2016. PMID: 27789538 Free PMC article. Review. - Combined CD28 and 4-1BB Costimulation Potentiates Affinity-tuned Chimeric Antigen Receptor-engineered T Cells.
Drent E, Poels R, Ruiter R, van de Donk NWCJ, Zweegman S, Yuan H, de Bruijn J, Sadelain M, Lokhorst HM, Groen RWJ, Mutis T, Themeli M. Drent E, et al. Clin Cancer Res. 2019 Jul 1;25(13):4014-4025. doi: 10.1158/1078-0432.CCR-18-2559. Epub 2019 Apr 12. Clin Cancer Res. 2019. PMID: 30979735 Free PMC article. - Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells.
You F, Jiang L, Zhang B, Lu Q, Zhou Q, Liao X, Wu H, Du K, Zhu Y, Meng H, Gong Z, Zong Y, Huang L, Lu M, Tang J, Li Y, Zhai X, Wang X, Ye S, Chen D, Yuan L, Qi L, Yang L. You F, et al. Sci China Life Sci. 2016 Apr;59(4):386-97. doi: 10.1007/s11427-016-5024-7. Epub 2016 Mar 7. Sci China Life Sci. 2016. PMID: 26961900 Clinical Trial. - Adnectin-Based Design of Chimeric Antigen Receptor for T Cell Engineering.
Han X, Cinay GE, Zhao Y, Guo Y, Zhang X, Wang P. Han X, et al. Mol Ther. 2017 Nov 1;25(11):2466-2476. doi: 10.1016/j.ymthe.2017.07.009. Epub 2017 Jul 20. Mol Ther. 2017. PMID: 28784559 Free PMC article. - T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance.
Chandran SS, Klebanoff CA. Chandran SS, et al. Immunol Rev. 2019 Jul;290(1):127-147. doi: 10.1111/imr.12772. Immunol Rev. 2019. PMID: 31355495 Free PMC article. Review.
Cited by
- CAR-NK cells in combination therapy against cancer: A potential paradigm.
Li J, Hu H, Lian K, Zhang D, Hu P, He Z, Zhang Z, Wang Y. Li J, et al. Heliyon. 2024 Feb 29;10(5):e27196. doi: 10.1016/j.heliyon.2024.e27196. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38486782 Free PMC article. Review. - FLT3L-induced virtual memory CD8 T cells engage the immune system against tumors.
Tu HF, Kung YJ, Lim L, Tao J, Hu MH, Cheng M, Xing D, Wu TC, Hung CF. Tu HF, et al. J Biomed Sci. 2024 Jan 29;31(1):19. doi: 10.1186/s12929-024-01006-9. J Biomed Sci. 2024. PMID: 38287325 Free PMC article. - Unlocking the potential of Tregs: innovations in CAR technology.
Requejo Cier CJ, Valentini N, Lamarche C. Requejo Cier CJ, et al. Front Mol Biosci. 2023 Oct 12;10:1267762. doi: 10.3389/fmolb.2023.1267762. eCollection 2023. Front Mol Biosci. 2023. PMID: 37900916 Free PMC article. Review. - The pleiotropic roles of EZH2 in T-cell immunity and immunotherapy.
Wang Y, Bui T, Zhang Y. Wang Y, et al. Int J Hematol. 2022 Dec;116(6):837-845. doi: 10.1007/s12185-022-03466-x. Epub 2022 Oct 21. Int J Hematol. 2022. PMID: 36271224 Review. - AKT Isoforms in the Immune Response in Cancer.
Ahmad Z, Somanath PR. Ahmad Z, et al. Curr Top Microbiol Immunol. 2022;436:349-366. doi: 10.1007/978-3-031-06566-8_15. Curr Top Microbiol Immunol. 2022. PMID: 36243852
References
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood. 2012;119(12):2709–2720. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA136551/CA/NCI NIH HHS/United States
- P50 CA138293/CA/NCI NIH HHS/United States
- R01 AI053193/AI/NIAID NIH HHS/United States
- R01 CA114536/CA/NCI NIH HHS/United States
- R01 CA136551/CA/NCI NIH HHS/United States
- AI053193/AI/NIAID NIH HHS/United States
- P50CA138293/CA/NCI NIH HHS/United States
- CA114536/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources