Zn²⁺ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation - PubMed (original) (raw)
. 2014 Jun 1;23(11):2791-801.
doi: 10.1093/hmg/ddt572. Epub 2013 Dec 13.
Affiliations
- PMID: 24334770
- PMCID: PMC4014186
- DOI: 10.1093/hmg/ddt572
Zn²⁺ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation
Taiji Tsunemi et al. Hum Mol Genet. 2014.
Abstract
Mutations in ATP13A2 (PARK9) cause Kufor-Rakeb syndrome (KRS) characterized by juvenile-onset parkinsonism, pyramidal signs and dementia. PARK9 belongs to type 5 P-type ATPase with its putative function as a cation transporter. Loss of PARK9 leads to lysosomal dysfunction and subsequent α-synuclein (α-Syn) accumulation. However, the mechanistic link between PARK9 and lysosomal dysfunction remains unclear. Here, we found that patient fibroblasts expressing mutant PARK9 or primary neurons with silenced PARK9 exhibited increased sensitivity to extracellular zinc (Zn(2+)). This effect was rescued with the Zn(2+) chelators clioquinol or TPEN. PARK9-deficient cells showed decreased lysosomal sequestration of Zn(2+) and increased expression of zinc transporters. Importantly, increased concentrations of Zn(2+) (Zn(2+) stress) resulted in lysosomal dysfunction that was partially restored by expression of wild-type PARK9. Zn(2+) stress also caused increased expression of α-Syn and consequently decreased activity of the lysosomal enzyme glucocerebrosidase. Together, these data suggest that PARK9 loss of function leads to dyshomeostasis of intracellular Zn(2+) that in turn contributes to lysosomal dysfunction and accumulation of α-Syn. It will be of interest to examine whether therapeutic lowering of zinc may prove beneficial for patients with KRS.
Figures
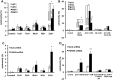
Figure 1.
PARK9 mutant fibroblasts and primary cortical neurons are sensitive to zinc (Zn2+). (A) LDH release from fibroblasts cultured in a medium containing one of the cations (Cu2+, Fe2+, Mn2+, Ni2+ or Zn2+, concentration is 100 μ
m
) for 24 h (n = 3, *P < 0.005, **P < 0.001). (B) Toxicity of Zn2+ was attenuated by adding Zn2+ chelator, clioquinol (2 μ
m
) in a medium (n = 3, *P < 0.01, **P < 0.03, ***P < 0.001). (C) LDH release from primary cortical neurons (PCN) cultured in the presence of cations (n = 3, *P < 0.005). (D) Toxicity of Zn2+ was attenuated by adding Zn2+ chelator, TPEN (N,N,_N_‘,N'-Tetrakis(2-pyridylmethyl)ethylenediamine) (1 μ
m
) (n = 3, *P < 0.05, **P < 0.005). The values are the mean ± SEM.
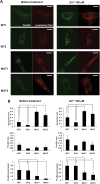
Figure 2.
PARK9 mutation alters Zn2+ distribution in fibroblasts. (A) The representative confocal live-cell images of fibroblasts stained with FluoZin-3 (green) and LysoTracker Red (red). Two wild-type (WT1 and WT2) and two PARK9 mutant (MUT1 and MUT2) fibroblast lines were stained before (left) or after treatment with 100 μ
m
Zn2+ for 1 h (right). Scale bars indicate 20 μm. (B) Quantification analysis of LysoTracker Red and FluoZin-3 area per total cell area before (left) and after Zn2+ incubation (right). Left, upper, quantification analysis of LysoTracker Red-positive area per total cell area (n = 10, *P < 0.01, **P < 0.001). Middle, quantification of FluoZin-3-positive area per total cell area (n = 10, *P < 0.005). Bottom, quantification of FluoZin-3-positive area per LysoTracker Red-positive area (n = 10, *P < 0.05). Right, upper, quantification of LysoTracker Red-positive area per total cell area (n = 10, *P < 0.05, **P < 0.03). Middle, quantification of FluoZin-3-positive area per total cell area. Bottom, quantification analysis of FluoZin-3-positive area per LysoTracker Red-positive area (n = 10, *P < 0.03, **P < 0.005). The values are the mean ± SEM.
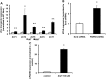
Figure 3.
Loss of PARK9 induces expression of zinc transporters and metallothionein proteins. (A) The mRNA expression of indicated zinc transporters in WT and MUT fibroblasts (n = 3, *P < 0.05, **P < 0.03). The expression of zinc transporters in MUT fibroblasts is divided by that in WT fibroblasts. (B) The mRNA expression of MT-III in scrambled- (Scrb) and PARK9 shRNA-treated primary cortical neurons (PCNs) (n = 3, *P < 0.001). The expression of MT-III in PARK9-treated PCNs is divided by that in Scrb-treated PCNs. (C) The PARK9 mRNA expression in PCNs cultured in the presence of 100 μ
m
Zn2+ (n = 3, *P < 0.001). The expression of PARK9 in PCNs cultured the medium containing 100 μ
m
Zn2+ is divided by that in PCNs cultured the normal medium. The values are the mean ± SEM.
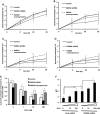
Figure 4.
Zinc dyshomeostasis leads to impaired lysosomal function. (A–E) PARK9 expression affects lysosomal proteolysis that is impaired by Zn2+. Lysosomal proteolysis in control, PARK9-depleted or PARK9 over-expressed PCNs cultured in a medium containing either 0 μ
m
(A), 1 μ
m
(B), 10 μ
m
(C) or 100 μ
m
Zn2+ (D). Lysosomal proteolysis is calculated by subtracting lysosomal inhibitors (2.5 m
m
NH4Cl and 50 μ
m
leupeptin) sensitive proteolysis from total proteolysis at 8, 20 and 28 h after chase. (E) Quantification of lysosomal proteolysis is calculated from the area under the curve (n = 3, *P < 0.05, **P < 0.005). (F) Dual-emission ratiometric measurement of lysosomal pH using LysoSensor Yellow/Blue DND-160. The effect of Zn2+ on lysosomal pH in Scrb or PARK9 shRNA treated PCNs is shown (n = 3, *P < 0.05, **P < 0.005). The values are the mean ± SEM.
Figure 5.
Loss of PARK9 enhances zinc-mediated α-Syn accumulation and reduction of lysosomal hydrolases activities. (A) Upper left: western blot analysis of the effect of Zn2+ on α-Syn expression in PCNs treated with Scrb or PARK9 shRNA. NSE was used as loading control. MW is shown as kDa. Bottom left: the results of densitometric analysis of α-Syn oligomers which were detected with syn 505 (*P < 0.01). Upper right: the results of densitometric analysis of α-Syn monomers which were detected with C-20 (*P < 0.01). (B) Glucocerebrosidase (GCase) activities in lysosome-enriched fractions extracted from PCNs (n = 3, *P < 0.005). (C) Acid sphingomyelinase (aSMase) activities in lysosome-enriched fractions extracted from PCNs (n = 3, *P < 0.03).
Similar articles
- Hereditary Parkinsonism-Associated Genetic Variations in PARK9 Locus Lead to Functional Impairment of ATPase Type 13A2.
Park JS, Sue CM. Park JS, et al. Curr Protein Pept Sci. 2017;18(7):725-732. doi: 10.2174/1389203717666160311121534. Curr Protein Pept Sci. 2017. PMID: 26965689 Review. - ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein.
Tsunemi T, Hamada K, Krainc D. Tsunemi T, et al. J Neurosci. 2014 Nov 12;34(46):15281-7. doi: 10.1523/JNEUROSCI.1629-14.2014. J Neurosci. 2014. PMID: 25392495 Free PMC article. - Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity.
Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Usenovic M, et al. J Neurosci. 2012 Mar 21;32(12):4240-6. doi: 10.1523/JNEUROSCI.5575-11.2012. J Neurosci. 2012. PMID: 22442086 Free PMC article. - Parkinson's disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction.
Park JS, Koentjoro B, Veivers D, Mackay-Sim A, Sue CM. Park JS, et al. Hum Mol Genet. 2014 Jun 1;23(11):2802-15. doi: 10.1093/hmg/ddt623. Epub 2014 Jan 7. Hum Mol Genet. 2014. PMID: 24399444 Free PMC article. - The role of ATP13A2 in Parkinson's disease: Clinical phenotypes and molecular mechanisms.
Park JS, Blair NF, Sue CM. Park JS, et al. Mov Disord. 2015 May;30(6):770-9. doi: 10.1002/mds.26243. Epub 2015 Apr 21. Mov Disord. 2015. PMID: 25900096 Review.
Cited by
- Unbiased Cell-based Screening in a Neuronal Cell Model of Batten Disease Highlights an Interaction between Ca2+ Homeostasis, Autophagy, and CLN3 Protein Function.
Chandrachud U, Walker MW, Simas AM, Heetveld S, Petcherski A, Klein M, Oh H, Wolf P, Zhao WN, Norton S, Haggarty SJ, Lloyd-Evans E, Cotman SL. Chandrachud U, et al. J Biol Chem. 2015 Jun 5;290(23):14361-80. doi: 10.1074/jbc.M114.621706. Epub 2015 Apr 15. J Biol Chem. 2015. PMID: 25878248 Free PMC article. - Cryo-EM structures and transport mechanism of human P5B type ATPase ATP13A2.
Chen X, Zhou M, Zhang S, Yin J, Zhang P, Xuan X, Wang P, Liu Z, Zhou B, Yang M. Chen X, et al. Cell Discov. 2021 Nov 2;7(1):106. doi: 10.1038/s41421-021-00334-6. Cell Discov. 2021. PMID: 34728622 Free PMC article. - Copper and Zinc Homeostasis: Lessons from Drosophila melanogaster.
Navarro JA, Schneuwly S. Navarro JA, et al. Front Genet. 2017 Dec 21;8:223. doi: 10.3389/fgene.2017.00223. eCollection 2017. Front Genet. 2017. PMID: 29312444 Free PMC article. Review. - Zinc Toxicity and Iron-Sulfur Cluster Biogenesis in Escherichia coli.
Li J, Ren X, Fan B, Huang Z, Wang W, Zhou H, Lou Z, Ding H, Lyu J, Tan G. Li J, et al. Appl Environ Microbiol. 2019 Apr 18;85(9):e01967-18. doi: 10.1128/AEM.01967-18. Print 2019 May 1. Appl Environ Microbiol. 2019. PMID: 30824435 Free PMC article. - Key genes and convergent pathogenic mechanisms in Parkinson disease.
Coukos R, Krainc D. Coukos R, et al. Nat Rev Neurosci. 2024 Jun;25(6):393-413. doi: 10.1038/s41583-024-00812-2. Epub 2024 Apr 10. Nat Rev Neurosci. 2024. PMID: 38600347 Review.
References
- Najim al-Din A.S., Wriekat A., Mubaidin A., Dasouki M., Hiari M. Pallido-pyramidal degeneration, supranuclear upgaze paresis and dementia: Kufor-Rakeb syndrome. Acta Neurol. Scand. 1994;89:347–352. - PubMed
- Ramirez A., Heimbach A., Grundemann J., Stiller B., Hampshire D., Cid L.P., Goebel I., Mubaidin A.F., Wriekat A.L., Roeper J., et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 2006;38:1184–1191. - PubMed
- Park J.S., Mehta P., Cooper A.A., Veivers D., Heimbach A., Stiller B., Kubisch C., Fung V.S., Krainc D., Mackay-Sim A., et al. Pathogenic effects of novel mutations in the P-type ATPase ATP13A2 (PARK9) causing Kufor-Rakeb syndrome, a form of early-onset parkinsonism. Hum. Mutat. 2011;32:956–964. - PubMed
- Ramonet D., Podhajska A., Stafa K., Sonnay S., Trancikova A., Tsika E., Pletnikova O., Troncoso J.C., Glauser L., Moore D.J. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum. Mol. Genet. 2012;21:1725–1743. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous