Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells - PubMed (original) (raw)
. 2014 Jan 21;111(3):996-1001.
doi: 10.1073/pnas.1317788111. Epub 2013 Dec 13.
Jesse R Dixon, Michael I J A van der Reijden, Zhen Ye, Petros Kolovos, Rutger W W Brouwer, Mariëtte P C van de Corput, Harmen J G van de Werken, Tobias A Knoch, Wilfred F J van IJcken, Frank G Grosveld, Bing Ren, Kerstin S Wendt
Affiliations
- PMID: 24335803
- PMCID: PMC3903193
- DOI: 10.1073/pnas.1317788111
Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells
Jessica Zuin et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Recent studies of genome-wide chromatin interactions have revealed that the human genome is partitioned into many self-associating topological domains. The boundary sequences between domains are enriched for binding sites of CTCC-binding factor (CTCF) and the cohesin complex, implicating these two factors in the establishment or maintenance of topological domains. To determine the role of cohesin and CTCF in higher-order chromatin architecture in human cells, we depleted the cohesin complex or CTCF and examined the consequences of loss of these factors on higher-order chromatin organization, as well as the transcriptome. We observed a general loss of local chromatin interactions upon disruption of cohesin, but the topological domains remain intact. However, we found that depletion of CTCF not only reduced intradomain interactions but also increased interdomain interactions. Furthermore, distinct groups of genes become misregulated upon depletion of cohesin and CTCF. Taken together, these observations suggest that CTCF and cohesin contribute differentially to chromatin organization and gene regulation.
Keywords: 4C; HOX cluster; Hi-C; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
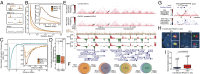
Cohesin cleavage reduces long-range interactions within the _H19/IGF2 domain. (A) Scheme of the expression construct. (B) Outline of the experiment. (C) Time course showing full cleavage of RAD21cv 24 h after HRV transfection. (D) Fractionation of uninduced cells (−dox), and transfected (TEV or HRV) RAD21cv cells into soluble and chromatin-bound fraction. Blotting for RAD21 shows full replacement of endogenous RAD21 by RAD21cv after induction (+dox). Detection of the RAD21cv C-terminal EGFP-tag shows full cleavage of RAD21cv after HRV transfection (+dox, HRV) and release of RAD21cv as well as the cohesin subunits STAG1 and STAG2 (SA1/2) from chromatin. CTCF binding to chromatin is not affected. (E) ChIP-qPCR with anti-EGFP targeting the RAD21cv EGFP-tag shows a reduced ChIP signal after HRV transfection at cohesin sites. (F–H) The effect of RAD21 cleavage on long-range interactions was tested by 4C at six different viewpoints in two topological domains of chromosome 11. (F) Domain identification in IMR90 cells (domain boundaries, blue boxes) (1). (G) Cohesin sites (SMC3) determined in control cells. Primer pairs used for qPCR in E are indicated. (H) The 4C interaction profiles for six different viewpoints (highlighted in green) without (RAD21cv/TEV) and with RAD21 cleavage (RAD21cv/HRV). Data are displayed as reads per million (RPM) and only interactions above a cutoff based on P value < 0.05 are displayed.
Fig. 2.
Cohesin cleavage reduces interactions within topological domains. (A) Stratification of the Hi-C interaction map based on SMC3 binding. Clustering of interacting 40-kb bins for presence of SMC3 (brown peak) at both interacting bins (2×), at one bin (1×) or no SMC3 (none). (B) The normalized interaction frequency is plotted versus the distance of interacting bins for the different bin clusters (SMC3 2×, 1×, and none). (Inset) In the fold-change relative to the “none” category the SMC3 2× cluster has highest interactions frequencies. (C) Cohesin destruction reduces the interactions between bins. The change of interaction frequency after RAD21 cleavage (HRV-TEV, dark-green curve) is plotted relative to the distance between interacting bins and reveals a reduction of interactions at distances up to 4 Mb. (Inset) The loss of interactions for the SMC3 2×, 1×, and none categories. (D) Interaction frequencies are significantly reduced after RAD21 cleavage, shown here at the example of bins 240-kb apart. (E) Normalized Hi-C interaction frequencies observed in RAD21cv cells transfected with either TEV or HRV protease are shown. SMC3 ChIP-sequencing, topological domains positions (DC, domain calls) and directionality index (DI) are shown. Arrows indicate regions with significant changes. (F) Comparison of topological domain boundary calls between Hi-C replicates (TEV1/TEV2; HRV1/HRV2) and control (TEV) and RAD21-depleted cells (HRV). Variations between the respective replicates and between control and RAD21 cleavage experiments are comparable, indicating that the number of domains does not change. (G) Position of cosmid-based DNA-FISH probes at the topological domain including the HOXD locus. The color of the cosmid probes (red, green) corresponds to the DNA-FISH images in (H). Arrows mark the interactions visualized by DNA-FISH. (H) DNA-FISH using the cosmid probes shown in G in control cells (TEV) and after RAD21 cleavage (HRV). The marked DNA-FISH signals (white boxes) are shown enlarged at the right side of each panel. Consistent with the Hi-C experiments, we observe separation of the FISH signals after RAD21 cleavage. (I) Distances between the FISH-probes observed in the TEV and HRV experiments. The P value was calculated using an ANOVA test on the log distances.
Fig. 3.
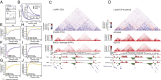
CTCF depletion reduces the function of domain boundaries. (A) As in Fig.2_A_, interacting 40-kb bins were analyzed for the presence of CTCF at one or both interacting bins. (B) The normalized interaction frequency was plotted versus the distance between bins for each class of interactions (CTCF 2×, CTCF 1×, and none). (Inset) In the fold-change relative to the “none” category, the CTCF 2× class has a higher interaction frequency than CTCF 1× with a maximum for bins 100- to 200-kb apart. (C) The differential interaction map (HRV-TEV) displays changes in interaction frequencies after RAD21 cleavage (red, gain of interactions; blue, loss of interactions). RAD21 depletion leads predominantly to reduced interactions within domains. The domain identification (domain calls, DC; directionality index, DI) is also shown. (D) Similar to C, but showing the changes in interaction frequencies at the same region after CTCF depletion by siRNA. A similar pattern of reduced intradomain interaction frequency as in C is observed, visible as blue outline of the domains in the differential plot (siCTCF-siControl). CTCF depletion yields increased interdomain interactions, visible as red signals between domains. (E) Quantification of the average change of interaction frequencies after RAD21 depletion, analyzed separately for intradomain (blue) and interdomain (yellow) interactions. In both cases, RAD21 depletion leads to a reduced interaction frequency. (F and G) The frequency change of intra- (F) and interdomain (G) interactions was analyzed for the presence of SMC3 on the interacting bins. The loss of interactions is in both cases correlated with SMC3-binding (F, purple; G, orange). (H) Quantification of the average change of interaction frequencies after CTCF siRNA depletion separated for intradomain (blue) and interdomain (yellow) interactions. CTCF depletion leads to a reduced interaction frequency within and to an increased interaction frequency between domains. (I and J) The change of interaction frequency of intra- (I) and interdomain (J) interactions was further analyzed for the presence of CTCF on the interacting bins. The gain of interactions is more pronounced for interdomain interactions (J) and is stronger when CTCF-sites are present in the interacting bins (J, orange).
Fig. 4.
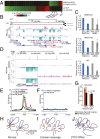
Transcriptional changes after cohesin cleavage and CTCF depletion. (A) Changes in expression levels after RAD21 cleavage (Upper) and CTCF depletion (Lower) (FDR < 0.05) are ranked from highest to lowest. Only very few genes behave similarly in both experiments. (B) Expression of HOXA genes changes after RAD21 cleavage. Normalized RNA-seq read coverage is shown for RAD21cv/TEV and RAD21cv/HRV cells (+strand, purple; −strand, turquoise). HOX genes differentially expressed with FDR < 0.2 are marked in red. (C) qPCR confirmation of reduced HOXB-AS3 and HOXA-AS3 expression after RAD21 cleavage. CTCF depletion did not lead to a consistent reduction, as also seen in the analysis of the RNA-seq data (
SI Appendix, Table S1
). Transcription of the H19 noncoding RNA was reduced after CTCF depletion and also by RAD21 cleavage. (mean n = 3 ± SD). (D) Transcription of the ENPP3 gene is increased after CTCF knockdown. Normalized RNA-seq read coverage are shown for control siRNA and CTCF siRNA (+strand, purple; −strand, turquoise). CTCF binding sites are at the promoter and also intragenic. The up-regulation was confirmed by RT-PCR to depend solely on CTCF knockdown (
SI Appendix, Fig. S9_F_
). (E) Position of CTCF sites analyzed relative to transcription start sites of all genes (black) and genes with altered expression after CTCF depletion (blue). Each line represents the average fold-enrichment of CTCF relative to input over a ±2.5-kb window surrounding the promoters of genes bound by CTCF. CTCF is enriched at the transcription start site of differentially expressed, in particular down-regulated genes (green), but it localizes more in the gene body at up-regulated genes. (F) Similar to E, except showing the fold-enrichment of SMC3 over input at the promoter of genes altered after RAD21 cleavage. SMC3 does not appear to be enriched at the promoter of the genes regulated by cohesin depletion. (G) Analysis of changes in interaction frequency between restriction fragments containing a promoter and restriction fragments containing a distal DNaseI hypersensitive site (DHS). Shown is the fraction of genes that display a 50% reduction or 50% increase in interaction frequency after RAD21 cleavage for either cohesin-regulated genes (orange) or all Ref-seq genes (black). Cohesin-regulated genes are enriched for a loss of interactions with restriction fragments containing distal DHS sites relative to all Ref-seq genes (Fisher’s exact test). (H–J), Models describing the different changes of chromosomal interactions after cohesin cleavage (I) and CTCF depletion (J). (H) Cohesin and CTCF shape long-range interactions. (I) RAD21 cleavage destroys cohesin and leads to reduced interactions within domains. CTCF binding does not change and can still influence chromatin topology and maintain domain identity. (J) CTCF depletion leads to more dynamic domains and interactions across domain boundaries, normally prevented by CTCF’s insulation function, potentially involving nonspecifically localizing cohesin.
Comment in
- CTCF and cohesin cooperate to organize the 3D structure of the mammalian genome.
Baranello L, Kouzine F, Levens D. Baranello L, et al. Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):889-90. doi: 10.1073/pnas.1321957111. Epub 2014 Jan 7. Proc Natl Acad Sci U S A. 2014. PMID: 24398527 Free PMC article. No abstract available.
Similar articles
- Exploring CTCF and cohesin related chromatin architecture at HOXA gene cluster in primary human fibroblasts.
Wang X, Xu M, Zhao G, Liu G, Hao D, Lv X, Liu D. Wang X, et al. Sci China Life Sci. 2015 Sep;58(9):860-6. doi: 10.1007/s11427-015-4913-5. Epub 2015 Sep 16. Sci China Life Sci. 2015. PMID: 26376810 - Genome-wide studies of CCCTC-binding factor (CTCF) and cohesin provide insight into chromatin structure and regulation.
Lee BK, Iyer VR. Lee BK, et al. J Biol Chem. 2012 Sep 7;287(37):30906-13. doi: 10.1074/jbc.R111.324962. Epub 2012 Sep 5. J Biol Chem. 2012. PMID: 22952237 Free PMC article. Review. - Cell cycle control of Kaposi's sarcoma-associated herpesvirus latency transcription by CTCF-cohesin interactions.
Kang H, Lieberman PM. Kang H, et al. J Virol. 2009 Jun;83(12):6199-210. doi: 10.1128/JVI.00052-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369356 Free PMC article. - Tissue-specific CTCF-cohesin-mediated chromatin architecture delimits enhancer interactions and function in vivo.
Hanssen LLP, Kassouf MT, Oudelaar AM, Biggs D, Preece C, Downes DJ, Gosden M, Sharpe JA, Sloane-Stanley JA, Hughes JR, Davies B, Higgs DR. Hanssen LLP, et al. Nat Cell Biol. 2017 Aug;19(8):952-961. doi: 10.1038/ncb3573. Epub 2017 Jul 24. Nat Cell Biol. 2017. PMID: 28737770 Free PMC article. - CTCF as a boundary factor for cohesin-mediated loop extrusion: evidence for a multi-step mechanism.
Hansen AS. Hansen AS. Nucleus. 2020 Dec;11(1):132-148. doi: 10.1080/19491034.2020.1782024. Nucleus. 2020. PMID: 32631111 Free PMC article. Review.
Cited by
- SMARCA4 regulates gene expression and higher-order chromatin structure in proliferating mammary epithelial cells.
Barutcu AR, Lajoie BR, Fritz AJ, McCord RP, Nickerson JA, van Wijnen AJ, Lian JB, Stein JL, Dekker J, Stein GS, Imbalzano AN. Barutcu AR, et al. Genome Res. 2016 Sep;26(9):1188-201. doi: 10.1101/gr.201624.115. Epub 2016 Jul 19. Genome Res. 2016. PMID: 27435934 Free PMC article. - A cohesin-OCT4 complex mediates Sox enhancers to prime an early embryonic lineage.
Abboud N, Morris TM, Hiriart E, Yang H, Bezerra H, Gualazzi MG, Stefanovic S, Guénantin AC, Evans SM, Pucéat M. Abboud N, et al. Nat Commun. 2015 Apr 8;6:6749. doi: 10.1038/ncomms7749. Nat Commun. 2015. PMID: 25851587 Free PMC article. - Pairing and anti-pairing: a balancing act in the diploid genome.
Joyce EF, Erceg J, Wu CT. Joyce EF, et al. Curr Opin Genet Dev. 2016 Apr;37:119-128. doi: 10.1016/j.gde.2016.03.002. Epub 2016 Apr 9. Curr Opin Genet Dev. 2016. PMID: 27065367 Free PMC article. Review. - ChIPr: accurate prediction of cohesin-mediated 3D genome organization from 2D chromatin features.
Abbas A, Chandratre K, Gao Y, Yuan J, Zhang MQ, Mani RS. Abbas A, et al. Genome Biol. 2024 Jan 12;25(1):15. doi: 10.1186/s13059-023-03158-7. Genome Biol. 2024. PMID: 38217027 Free PMC article. - Organization of nuclear architecture during adipocyte differentiation.
Charó NL, Rodríguez Ceschan MI, Galigniana NM, Toneatto J, Piwien-Pilipuk G. Charó NL, et al. Nucleus. 2016 May 3;7(3):249-69. doi: 10.1080/19491034.2016.1197442. Nucleus. 2016. PMID: 27416359 Free PMC article. Review.
References
- Wendt KS, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451(7180):796–801. - PubMed
- Parelho V, et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132(3):422–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases