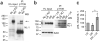CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses - PubMed (original) (raw)
CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses
Michael A Bemben et al. Nat Neurosci. 2014 Jan.
Abstract
Neuroligins are postsynaptic cell adhesion molecules that are important for synaptic function through their trans-synaptic interaction with neurexins (NRXNs). The localization and synaptic effects of neuroligin-1 (NL-1, also called NLGN1) are specific to excitatory synapses with the capacity to enhance excitatory synapses dependent on synaptic activity or Ca(2+)/calmodulin kinase II (CaMKII). Here we report that CaMKII robustly phosphorylates the intracellular domain of NL-1. We show that T739 is the dominant CaMKII site on NL-1 and is phosphorylated in response to synaptic activity in cultured rodent neurons and sensory experience in vivo. Furthermore, a phosphodeficient mutant (NL-1 T739A) reduces the basal and activity-driven surface expression of NL-1, leading to a reduction in neuroligin-mediated excitatory synaptic potentiation. To the best of our knowledge, our results are the first to demonstrate a direct functional interaction between CaMKII and NL-1, two primary components of excitatory synapses.
Figures
Figure 1
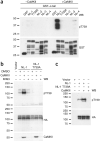
NL-1 T739 is phosphorylated by CaMKII in vitro. (a) Alignment of the transmembrane domains and c-tails of NL-1 (mouse), NL-2 (rat), NL-3 (human) and NL-4 (human). The CaMKII phosphorylation site boxed in red, and the pT739-Ab epitope is boxed in gray. (b–d) Audioradiography analysis of GST fusion proteins that were incubated with purified CaMKII, PKA or PKC and [γ-32P]ATP (P-32). The arrow denotes autophosphorylated PKA. Total protein was visualized by Coomassie Brilliant Blue (CBB) protein staining, and GST and the GST-GluA1 c-tail served as the negative and positive phosphorylation controls, respectively, in b–d. (e) Electron transfer dissociation MS/MS spectrum of the phosphorylated NL-1 peptide 730RRCSPQRTTpTNDLTHAPEEEIM751 (where p indicates phosphorylation) found only in GST–NL-1 fusion proteins incubated with ATP and purified CaMKII and not those incubated with PKA or PKC. Samples were digested with chymotrypsin and then analyzed using LC/MS/MS method. m/z, mass-to-charge ratio. (f) Extracted ion chromatogram of a quadruply charged ion at m/z 681.30, which corresponds to the phosphorylated NL-1730–751 peptide, as shown in e, for GST–NL-1 without enzyme (red), with PKA (gray), with PKC (green) or with CaMKII (blue). (g) Extracted ion chromatogram of a quadruply charged ion at m/z 661.31, which corresponds to the nonphosphorylated NL-1730–751 peptide in GST–NL-1 without enzyme (red), with PKA (gray), with PKC (green) or with CaMKII (blue). Full-length blots are presented in Supplementary Figure 4 when applicable.
Figure 2
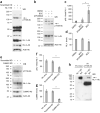
NL-1 T739 is phosphorylated by CaMKII in vitro and in hererologous cells as detected by a phosphorylation state–specific antibody. (a) Immunoblot analysis with pT739-Ab of GST, GST–NL-1 (wild type or T739A), GST–NL-2, GST–NL-3 and GST–NL-4 that were phosphorylated in vitro with purified catalytic subunits of CaMKII. Immunoblotting with GST-Ab confirmed equal loading of the protein. WB, western blot. (b) Immunoblot analysis of NL-1 (wild type or T739A) transfected in COS cells and treated with a CaMKII inhibitor, KN93, or cotransfected with constitutively active CaMKII (T286D). (c) Cotransfection of NL-1 (wild type or T739A) with CaMKII (T286D) in HEK293T cells. Immunoblots were probed with the antibodies indicated in b and c. Full-length blots are presented in Supplementary Figure 4 when applicable.
Figure 3
Phosphorylation of NL-1 T739 in neurons. (a) Detection of NL-1 T739 phosphorylation in DIV 21 cortical neurons with or without expression of a lentiviral shRNA, either scrambled (scrambled knockdown (KD)) or targeting NL-1 (NL-1 KD). (b) Regulation of NL-1 T739 phosphorylation under control conditions (DMSO) or in the presence of BCC with or without pretreatment of AP5 and NBQX (AP5/NBQX) in DIV 21 cortical neurons. Immunoblots were probed with the antibodies indicated. (c) Phosphorylated NL-1 (pNL-1) to total NL-1 (means ± s.e.m.) normalized to DMSO control (n = 7) with AP-5/NBQX pretreatment plus BCC (P > 0.05, n = 4) or with BCC treatment alone (P < 0.0006, _n_ = 7). (**d**) Total NL-1 (means ± s.e.m.) normalized to actin control (_n_ = 7) with AP5/NBQX pretreatment plus BCC (_P_ > 0.05, n = 4) or with BCC treatment alone (P = 0.0156, n = 7). (e) Detection of NL-1 T739 phosphorylation in DIV 21 cortical neurons with or without expression of a lentiviral shRNA, either scrambled or targeting CaMKII. (f) Phosphorylated to total NL-1 (means ± s.e.m.) normalized to no virus treatment (n = 4) with scrambled knockdown (P > 0.05, n = 4) or CaMKII knockdown (P = 0.0286, n = 4). (g) Total CaMKII (means ± s.e.m.) normalized to actin control (n = 4) with scrambled knockdown (P > 0.05, n = 4) or CaMKII knockdown (P = 0.029, n = 4). (h) Detection of NL-1 T739 phosphorylation in adult wild-type or NL-1 knockout (KO) brains. Arrows in a and b denote the NL-1–specific band. IP, immunoprecipitation. *P < 0.05, ***P < 0.001. Full-length blots are presented in Supplementary Figure 4 when applicable.
Figure 4
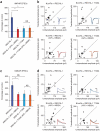
T739A reduces the surface expression and synaptic enhancement of NL-1. (a) Hippocampal neurons coexpressing NLmiRs and NL-1 (wild type or T739A). Surface receptors were labeled with anti-HA and Alexa 555–conjugated secondary antibody (red). After fixation and permeabilization, internal receptors were visualized with anti-HA and Alexa 647–conjugated secondary antibody (pseudocolored white) (scale bar, 50 μm). The images below each row are enlargements of the boxes areas. (b) NL-1 T739A levels (P = 0.0001, n = 36; means ± s.e.m.) normalized to NL-1 (n = 34). (c) Neurons transfected as in a. VGLUT1 was labeled with anti-VGLUT1 and Alexa 647–conjugated secondary antibody (pseudocolored white), and PSD-95 was visualized with anti–PSD-95 and Alexa 555–conjugated secondary antibody (red). The images in c are shown at the same magnification as the bottom rows in a. (d) VGLUT1 levels (means ± s.e.m.) normalized to NL-1 (n = 24) for NLmiRs (P = 0.0001, n = 23) and NL-1 T739A (P = 0.0001, n = 24). (e) PSD-95 levels (means ± s.e.m.) normalized to NL-1 (n = 24) for NLmiRs (P = 0.0001, n = 23) and NL-1 T739A (P = 0.0001, n = 24). (f) Colocalized VGLUT1 and PSD-95 puncta (means ± s.e.m.) normalized to NL-1 (n = 24) for NLmiRs (P = 0.0001, n = 23) and NL-1 T739A (P = 0.0182, n = 24). (g) Representative mEPSC traces of hippocampal neurons expressing GFP, NLmiRs or NLmiRs and NL-1 (wild type or T739A) (vertical scale bar, 15 pA; horizontal scale bar, 500 ms). (h) Spontaneous mEPSC mean amplitudes (error bars, s.e.m.) of cells transfected with GFP (n = 9), NLmiRs (P > 0.05, n = 9), NLmiRs plus NL-1 (P = 0.0028, n = 9) or NLmiRs plus NL-1 T739A (P = 0.0003, n = 9) as compared to GFP-transfected cells. NL-1 T739A had no change in mEPSC amplitude when compared to NL-1 (not significant (NS, P > 0.05), n = 9). (i) Spontaneous mEPSCs (mean frequencies ± s.e.m.) of cells transfected with GFP (n = 9), NLmiRs (P = 0.0031, n = 9), NLmiRs plus NL-1 (P = 0.0012, n = 9) or NLmiRs plus NL-1 T739A (P > 0.05, n = 9) as compared to GFP-transfected cells. NL-1 T739A reduces mEPSC frequency when compared to NL-1 (P = 0.0001, n = 9). *P < 0.05 **P < 0.01, ***P < 0.001.
Figure 5
Activity-dependent increase in NL-1 surface expression is diminished in NL-1 T739A. (a) Hippocampal neurons coexpressing NLmiRs and NL-1 (wild type or T739A), as in Figure 4a, that were treated for 2 h with DMSO or BCC. Scale bar, 25 μm. (b) Levels of NL-1 plus BCC (P = 0.0027, n = 34), NL-1 T739A + DMSO (n = 28) and NL-1 T739A + BCC (P > 0.05, n = 28) normalized to NL-1 + DMSO (n = 36) (means ± s.e.m.). **P < 0.01.
Figure 6
Synaptic enhancement by NL-1 is reduced by either glutamate receptor blockade or the T739A mutation. (a) Low-level postsynaptic expression of NL-1 (n = 17) results in an enhancement of AMPAR-mediated currents to a greater extent than does the expression of NL-1 in the presence of AP5 and NBQX (P = 0.0439, n = 15) or the expression of NL-1 T739A (P = 0.0470, n = 12). Expression of NL-1 T739A in the presence of AP5 and NBQX did not further reduce AMPAR currents (P > 0.05, n = 9). The data are plotted as the percentage of control (mean ± s.e.m.) comparing transfected cells to simultaneously recorded, neighboring untransfected cells. All expression is on the background of the NLmiRs. (b) Scatter plots showing the individual conditions summarized in a. Open circles represent individual paired recordings, and filled circles represent the means ± s.e.m. The traces show representative currents for each condition, with the transfected cell in color and the control cell in black (vertical scale bars, 30 pA; horizontal scale bars, 20 ms). (c) A similar result to that in a is shown for NMDAR-mediated currents, with a greater enhancement by low-level postsynaptic expression of NL-1 (n = 17) than by NL-1 expression in the presence of AP5 and NBQX (P = 0.0363, n = 15) or the expression of NL-1 T739A (P = 0.0599, n = 12). Expression of NL-1 T739A in the presence of AP5 and NBQX did not further reduce NMDAR currents (P > 0.05, n = 7). The bar graph is as described in a. (d) Scatter plots showing the individual conditions summarized in c. Open circles represent individual paired recordings, and filled circles represent the means ± s.e.m. Traces show representative currents for each condition, with the transfected cell in color and the control cell in black (vertical scale bars, 60 pA; horizontal scale bars, 100 ms). *P < 0.05.
Figure 7
Synaptic activity dynamically regulates T739 phosphorylation in vivo. (a) Detection of NL-1 T739 phosphorylation in adult wild-type or NL-1 knockout visual cortex. (b) Detection of NL-1 T739 phosphorylation in the visual cortex of wild-type mice that were either light reared for 26 d (LR), light reared to P21 and then subjected to 5 d of dark rearing from P21 to P26 (DR) or light reared and then subjected to 5 d of dark rearing (P21–P26) and finally exposed to 2 h of light stimulus at P26 (DR + LR). When animals were light reared, they were maintained on a normal day and night cycle, whereas dark-reared animals were in complete darkness for 5 d. Also shown is the regulation of NL-1 T739 phosphorylation in light-reared, dark-reared or dark-reared plus light-reared P26 visual cortices. Adult NL-1 knockout visual cortices served as negative controls, and actin served as a total protein control. Nonsaturating protein concentrations (0.25 mg) were used, as described in Supplementary Figure 3a,b. (c) pNL-1 (immunoprecipitated) to total NL-1 (input) (means ± s.e.m.) normalized to light-reared control (n = 7) for the dark-reared (P = 0.0156, n = 7) and dark-reared plus light-reared (P = 0.0469, n = 7) conditions. All comparisons between conditions were using littermates. *P < 0.05. Full-length blots are presented in Supplementary Figure 4 when applicable.
Similar articles
- Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2.
Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Südhof TC. Chubykin AA, et al. Neuron. 2007 Jun 21;54(6):919-31. doi: 10.1016/j.neuron.2007.05.029. Neuron. 2007. PMID: 17582332 Free PMC article. - AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors.
Sanderson JL, Gorski JA, Gibson ES, Lam P, Freund RK, Chick WS, Dell'Acqua ML. Sanderson JL, et al. J Neurosci. 2012 Oct 24;32(43):15036-52. doi: 10.1523/JNEUROSCI.3326-12.2012. J Neurosci. 2012. PMID: 23100425 Free PMC article. - Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors.
Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK. Lozada AF, et al. J Neurosci. 2012 May 30;32(22):7651-61. doi: 10.1523/JNEUROSCI.6246-11.2012. J Neurosci. 2012. PMID: 22649244 Free PMC article. - Neuroligin-3: A Circuit-Specific Synapse Organizer That Shapes Normal Function and Autism Spectrum Disorder-Associated Dysfunction.
Uchigashima M, Cheung A, Futai K. Uchigashima M, et al. Front Mol Neurosci. 2021 Oct 6;14:749164. doi: 10.3389/fnmol.2021.749164. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34690695 Free PMC article. Review. - [Proteolytic cleavage of neuroligins and functions of their cleavage products].
Yu J, Xu J. Yu J, et al. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 Aug 25;49(4):514-523. doi: 10.3785/j.issn.1008-9292.2020.08.11. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 32985166 Free PMC article. Review. Chinese.
Cited by
- Regulation of NLGN3 and the Synaptic Rho-GEF Signaling Pathway by CDK5.
Jeong J, Han W, Hong E, Pandey S, Li Y, Lu W, Roche KW. Jeong J, et al. J Neurosci. 2023 Nov 1;43(44):7264-7275. doi: 10.1523/JNEUROSCI.2309-22.2023. Epub 2023 Sep 12. J Neurosci. 2023. PMID: 37699715 Free PMC article. - Novel interactive partners of neuroligin 3: new aspects for pathogenesis of autism.
Shen C, Huo LR, Zhao XL, Wang PR, Zhong N. Shen C, et al. J Mol Neurosci. 2015 May;56(1):89-101. doi: 10.1007/s12031-014-0470-9. Epub 2014 Dec 3. J Mol Neurosci. 2015. PMID: 25464930 - Activity-dependent proteolytic cleavage of cell adhesion molecules regulates excitatory synaptic development and function.
Nagappan-Chettiar S, Johnson-Venkatesh EM, Umemori H. Nagappan-Chettiar S, et al. Neurosci Res. 2017 Mar;116:60-69. doi: 10.1016/j.neures.2016.12.003. Epub 2016 Dec 10. Neurosci Res. 2017. PMID: 27965136 Free PMC article. Review. - Tyrosine phosphorylation of the transmembrane protein SIRPα: Sensing synaptic activity and regulating ectodomain cleavage for synapse maturation.
Nagappan-Chettiar S, Johnson-Venkatesh EM, Umemori H. Nagappan-Chettiar S, et al. J Biol Chem. 2018 Aug 3;293(31):12026-12042. doi: 10.1074/jbc.RA117.001488. Epub 2018 Jun 18. J Biol Chem. 2018. PMID: 29914984 Free PMC article. - LTP requires postsynaptic PDZ-domain interactions with glutamate receptor/auxiliary protein complexes.
Sheng N, Bemben MA, Díaz-Alonso J, Tao W, Shi YS, Nicoll RA. Sheng N, et al. Proc Natl Acad Sci U S A. 2018 Apr 10;115(15):3948-3953. doi: 10.1073/pnas.1800719115. Epub 2018 Mar 26. Proc Natl Acad Sci U S A. 2018. PMID: 29581259 Free PMC article.
References
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr. Opin. Cell Biol. 2003;15:621–632. - PubMed
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous