Epigenetic regulation of sensory axon regeneration after spinal cord injury - PubMed (original) (raw)
Epigenetic regulation of sensory axon regeneration after spinal cord injury
Mattéa J Finelli et al. J Neurosci. 2013.
Abstract
Axon regeneration is hindered by a decline of intrinsic axon growth capability in mature neurons. Reversing this decline is associated with the induction of a large repertoire of regeneration-associated genes (RAGs), but the underlying regulatory mechanisms of the transcriptional changes are largely unknown. Here, we establish a correlation between diminished axon growth potential and histone 4 (H4) hypoacetylation. When neurons are triggered into a growth state, as in the conditioning lesion paradigm, H4 acetylation is restored, and RAG transcription is initiated. We have identified a set of target genes of Smad1, a proregenerative transcription factor, in conditioned DRG neurons. We also show that, during the epigenetic reprogramming process, histone-modifying enzymes work together with Smad1 to facilitate transcriptional regulation of RAGs. Importantly, targeted pharmacological modulation of the activity of histone-modifying enzymes, such as histone deacetylases, leads to induction of multiple RAGs and promotion of sensory axon regeneration in a mouse model of spinal cord injury. Our findings suggest epigenetic modulation as a potential therapeutic strategy to enhance axon regeneration.
Keywords: DRG neurons; Smad1; axon regeneration; conditioning lesion; epigenetic regulation; spinal cord injury.
Figures
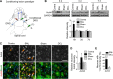
Figure 1.
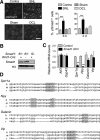
Global levels of AcH4 are increased in DRG neurons after peripheral axotomy. A, Schematic diagram of the conditioning lesion paradigm. DRG neurons (depicted as blue circles) have pseudounipolar axons with two branches: a peripheral branch innervating sensory targets (gray) and a central branch (blue) projecting into the spinal cord. The two branches react differently to axotomy: the peripheral branch can regenerate, but the central branch is refractory to regeneration. However, the central branch can be triggered into a growth state (2) if the peripheral branch is axotomized first (1), the so-called conditioning lesion. B, Western blot images and quantification of AcH4 levels in L4, L5, and L6 DRGs of mice 6 h, 3 d, and 7 d after sciatic nerve lesion (SNL, peripheral axotomy) or dorsal column lesion (DCL, central axotomy); contralateral DRGs (Contra) and DRGs from mice subjected to laminectomy (Sham) were used as controls, respectively (n = 6 independent experiments). C–E, Representative immunohistochemistry images (C) and quantification (D) of relative immunoreactivity of AcH4 in L5 DRG neurons (NeuN+) 6 h after a peripheral or a central lesion. A significantly increased percentage of sensory neurons in L5 DRGs exhibited increased nuclear AcH4 levels after a peripheral lesion (E). Scale bar, 50 μm. Arrows and arrowheads point to examples of increased nuclear AcH4 immunoreactivity in small- and large-diameter DRG neurons, respectively. Asterisks indicate glial cells in DRGs that did not show detectable changes in AcH4. Data represent mean ± SEM. *p < 0.05. ***p < 0.001.
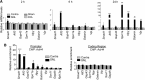
Figure 2.
AcH4 is specifically enriched at promoters of RAGs after peripheral axotomy. A, qRT-PCR of RAGs in mRNA extracts from L4–L6 DRGs 2, 6, or 24 h postlesion (n = 3 independent experiments). B, Quantitative PCR results of chromatin immunoprecipitated with AcH4 antibody at the promoters (left) or internal coding region (right) of RAGs and housekeeping genes (Gapdh or Rpl13a) in freshly dissected ipsilateral or contralateral L4–L6 DRGs from mice 6 h after sciatic nerve lesion (SNL). Relative enrichment of ChIP values is depicted as fold changes over contralateral control DRGs after normalization to input. Data represent mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001.
Figure 3.
HDAC inhibitor induces RAG transcription in sensory neurons. A, Western blot images and quantification of AcH4 levels in L4–L6 DRGs from vehicle, TSA, or MS-275-treated mice. B, C, Representative immunofluorescence images of DRGs from vehicle-, TSA-, or MS-275-treated mice with coimmunostaining of AcH4 (red) and neuronal markers β-tubulin (Tuj1) or NeuN (green). Scale bar, 100 μm. D, Quantitative PCR results of chromatin immunoprecipitated with AcH4 antibody at the promoter (left) or internal coding region (right) of RAGs and housekeeping genes (Gapdh and Rpl13a) in freshly dissected DRGs 6 h after the last of the 4 daily injections of vehicle or MS-275. E, qRT-PCR results of RAGs in mRNA extracts from DRGs of the same animals as in D (n = 3 independent experiments). F,G, qRT-PCR results of RAGs in mRNA extracts from DRGs of MS-275- or vehicle-treated mice (F). Of a total of 25 RAGs tested (18 shown in F plus 7 shown in E), 48% were not affected by MS-275 treatment (no change). A total of 40% exhibited same direction of transcriptional changes after MS-275 treatment compared with a conditioning lesion (concordant change), whereas 12% exhibited opposite direction of transcriptional changes (discordant change) (G). Data represent mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001.
Figure 4.
HDACi treatment enhances axon growth potential. A, Representative images of dissociated DRG neurons from ipsilateral (conditioned) versus contralateral (naive) L4–L6 DRGs of mice 6 d after sciatic nerve transection, or from vehicle- or HDACi-treated mice cultured for 48 h in vitro. Quantification of neurite outgrowth assay is presented as percentage increase of the mean maximal axonal length over respective control DRG neurons. Axon length was determined by β-tubulin (Tuj1) immunostaining and averaged from >100 randomly selected neurons each (n = 3 independent experiments). Scale bars: top, 200 μm; bottom, 100 μm. B, qPCR results of immunoprecipitated chromatin with AcH4 antibody from DRGs collected from mice 13 d after MS-275 treatment showed that AcH4 levels had returned to baseline at the RAG promoters (left). qRT-PCR analysis of RAGs in mRNA extracts from DRGs of the same mice (right). C, Quantification of neurite outgrowth from dissociated DRG neurons cultured for 24 or 48 h from vehicle- or MS-275-treated mice 13 d after treatment showed sustained axon growth capacity with MS-275 treatment. Axon length revealed by β-tubulin immunostaining was determined in >100 neurons for each condition and averaged. Data are mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001.
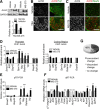
Figure 5.
Smad1 induces target genes in conditioned DRG neurons. A, Representative immunohistochemistry images and quantification of pSmad1+ neurons in L5 DRG 6 h and 3 d after either sciatic nerve (SNL) or dorsal column lesion (DCL), with control DRGs from contralateral (Contra) or mice with laminectomy only (Sham) (n = 3 mice each). Scale bar, 50 μm. B, Western blot using DRG lysates from Smad1 cKO mice or littermate controls probed for Smad1 or H3 (as loading control). C, qRT-PCR results of RAGs from mRNA extracted from freshly dissected DRGs from Smad1 cKO or littermate control mice. D, Human, mouse, and rat RAGs promoter sequences are aligned and conserved GC-rich SBEs are highlighted in gray. Data are mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001.
Figure 6.
Histone-modifying enzymes collaborate with Smad1 to regulate RAG expression. A–G, pSmad1 binding facilitates histone acetylation at the promoters of Smad1 target genes. qRT-PCR results of RAGs in mRNA extracts from Neuro-2A cells transfected with Smad1 siRNA for 48 h (A) or treated with BMP4 for 8 h (D); the controls were nontargeting siRNA or vehicle, respectively. B, C, E, F, Quantitative PCR results of pSmad1, AcH4-, HDAC1-, or p300-immunoprecipitated chromatin at promoters of the same RAGs and housekeeping genes. For the internal coding region, ChIP results using pSmad1, AcH4, HDAC1, or p300 antibodies all showed no change for the genes tested with Smad1 siRNA or BMP4 treatment, but for simplicity, only the AcH4 ChIP results are shown (G). H, I, H4 acetylation facilitates pSmad1 promoter occupancy in Smad1 target genes. qRT-PCR results of RAGs from mRNA extracts of Neuro-2A cells treated with MS-275 or vehicle for 48 h (H). Quantitative PCR results of chromatin immunoprecipitated with AcH4 or pSmad1 antibodies at the promoters of the RAGs and housekeeping genes in Neuro-2A cells (I). Relative enrichment of ChIP values is depicted as fold changes over controls after normalization to input. Data represent mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001. J, qRT-PCR results of RAGs from mRNA extracts from freshly cultured dissociated DRG neurons (2 h) from wild-type or Smad1 cKO mice treated with vehicle or MS-275. RAG induction by MS-275 was significantly attenuated for Sprr1a and Npy in mutant DRGs compared with wild-type DRGs. K, Coimmunoprecipitation with antibody against p300 in lysates of dissociated DRG neurons cultured for 3 d and immunoblotted for p300, Smad1, or GAPDH.
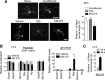
Figure 7.
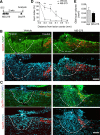
Sensory axon regeneration after SCI is enhanced with HDACi treatment. A, Experimental scheme. After SCI, animals were injected with either vehicle or HDACi daily for 4 consecutive days. Two weeks after SCI, regenerating axons were labeled using DexTR tracer and analyzed 3 d later. B, C, Representative images of sagittal sections of lesion centers of vehicle- or MS-275-treated mice. Ascending sensory axons were labeled with DexTR (red), and lesion borders (marked with dashed lines) were defined by a combination of dense GFAP or CSPG immunoreactivity (green) and DAPI nuclear counterstaining (blue) that demonstrates a gradient of increased cellular density from the lesion center (marked with asterisk). D, Axon index of DexTR+ axons showed that significantly more axons were found at −0.3, −0.2, and −0.1 mm caudal to the lesion center in MS275-treated mice than controls. E, Lesion volume at the spinal cord injury site defined by dense GFAP immunoreactivity (n = 7 pairs of mice). Scale bars: B, C, 100 μm. Data represent mean ± SEM. *p < 0.05. **p < 0.01. ***p < 0.001.
Figure 8.
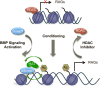
Working model. A conditioning lesion initiates epigenetic reprogramming that includes inducing and activating Smad1, removing HDACs, and recruiting HATs. A collaboration between pSmad1 promoter occupancy and histone acetylation helps to induce Smad1 target RAGs. Importantly, targeted modulation of the activity of this regeneration-associated transcriptional module via BMP signaling activation or pharmacological inhibition of HDACs can mimic the conditioning effect.
Similar articles
- Role of Myc Proto-Oncogene as a Transcriptional Hub to Regulate the Expression of Regeneration-Associated Genes following Preconditioning Peripheral Nerve Injury.
Shin HY, Kwon MJ, Lee EM, Kim K, Oh YJ, Kim HS, Hwang DH, Kim BG. Shin HY, et al. J Neurosci. 2021 Jan 20;41(3):446-460. doi: 10.1523/JNEUROSCI.1745-20.2020. Epub 2020 Dec 1. J Neurosci. 2021. PMID: 33262248 Free PMC article. - Genetic study of axon regeneration with cultured adult dorsal root ganglion neurons.
Saijilafu, Zhou FQ. Saijilafu, et al. J Vis Exp. 2012 Aug 17;(66):4141. doi: 10.3791/4141. J Vis Exp. 2012. PMID: 23117482 Free PMC article. - Exercise dependent increase in axon regeneration into peripheral nerve grafts by propriospinal but not sensory neurons after spinal cord injury is associated with modulation of regeneration-associated genes.
Sachdeva R, Theisen CC, Ninan V, Twiss JL, Houlé JD. Sachdeva R, et al. Exp Neurol. 2016 Feb;276:72-82. doi: 10.1016/j.expneurol.2015.09.004. Epub 2015 Sep 12. Exp Neurol. 2016. PMID: 26366525 Free PMC article. - Extrinsic and Intrinsic Regulation of Axon Regeneration by MicroRNAs after Spinal Cord Injury.
Li P, Teng ZQ, Liu CM. Li P, et al. Neural Plast. 2016;2016:1279051. doi: 10.1155/2016/1279051. Epub 2016 Oct 13. Neural Plast. 2016. PMID: 27818801 Free PMC article. Review. - Sensory axon regeneration: rebuilding functional connections in the spinal cord.
Smith GM, Falone AE, Frank E. Smith GM, et al. Trends Neurosci. 2012 Mar;35(3):156-63. doi: 10.1016/j.tins.2011.10.006. Epub 2011 Nov 30. Trends Neurosci. 2012. PMID: 22137336 Free PMC article. Review.
Cited by
- Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others?
Rodemer W, Hu J, Selzer ME, Shifman MI. Rodemer W, et al. Neural Regen Res. 2020 Jun;15(6):996-1005. doi: 10.4103/1673-5374.270298. Neural Regen Res. 2020. PMID: 31823869 Free PMC article. Review. - DLK regulates a distinctive transcriptional regeneration program after peripheral nerve injury.
Shin JE, Ha H, Kim YK, Cho Y, DiAntonio A. Shin JE, et al. Neurobiol Dis. 2019 Jul;127:178-192. doi: 10.1016/j.nbd.2019.02.001. Epub 2019 Feb 5. Neurobiol Dis. 2019. PMID: 30735704 Free PMC article. - BMP signaling in axon regeneration.
Zhong J, Zou H. Zhong J, et al. Curr Opin Neurobiol. 2014 Aug;27:127-34. doi: 10.1016/j.conb.2014.03.009. Epub 2014 Apr 10. Curr Opin Neurobiol. 2014. PMID: 24713578 Free PMC article. Review. - The Protein Acetylation after Traumatic Spinal Cord Injury: Mechanisms and Therapeutic Opportunities.
Li HW, Zhang HH. Li HW, et al. Int J Med Sci. 2024 Feb 12;21(4):725-731. doi: 10.7150/ijms.92222. eCollection 2024. Int J Med Sci. 2024. PMID: 38464830 Free PMC article. Review. - Molecular and Cellular Mechanisms of Axonal Regeneration After Spinal Cord Injury.
van Niekerk EA, Tuszynski MH, Lu P, Dulin JN. van Niekerk EA, et al. Mol Cell Proteomics. 2016 Feb;15(2):394-408. doi: 10.1074/mcp.R115.053751. Epub 2015 Dec 22. Mol Cell Proteomics. 2016. PMID: 26695766 Free PMC article. Review.
References
- Bosse F, Küry P, Müller HW. Gene expression profiling and molecular aspects in peripheral nerve regeneration. Restor Neurol Neurosci. 2001;19:5–18. - PubMed
- Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical