A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program - PubMed (original) (raw)
Review
A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program
Miguel O'Ryan et al. Drugs. 2014 Jan.
Abstract
Recently approved in Europe and Australia, the multi-component meningococcal B vaccine, 4CMenB (Bexsero®, Novartis Vaccines and Diagnostics), contains three surface-exposed recombinant proteins (fHbp, NadA, and NHBA) and New Zealand strain outer membrane vesicles (NZ OMV) with PorA 1.4 antigenicity. This comprehensive review of the 4CMenB clinical development program covers pivotal phase I/IIb/III studies in over 7,000 adults, adolescents, and infants. The immunological correlate for clinical protection used was human complement-mediated serum bactericidal activity titers ≥4 or 5 against indicator strains for individual antigens. Based on achievement of protective titers, a four-dose schedule (three primary doses and one booster dose) for infants and a two-dose schedule for adolescents provided the best results. Observed increases in injection site pain/tenderness and fever in infants, and injection site pain, malaise, and headache in adolescents compared with routine vaccines, were mostly mild to moderate; frequencies of rare events (Kawasaki disease, juvenile arthritis) were not significantly different from non-vaccinated individuals. 4CMenB is conservatively estimated to provide 66-91 % coverage against meningococcal serogroup B strains worldwide.
Figures
Fig. 1
Representation of the antigenic components of 4CMenB. 4CMenB contains three recombinant antigens, fHbp, NadA, and NHBA, combined with OMV from MenB strain NZ 98/254. fHbp factor H-binding protein, NadA Neisserial adhesin A, NHBA Neisseria heparin-binding antigen, OMV outer membrane vesicles, PorA-OMV NZ Porin A as part of the New Zealand strain OMV
Fig. 2
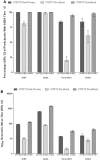
Immunogenicity in response to four doses of 4CMenB in infants. a Percentages of participants with hSBA titer ≥5. b Geometric mean titers. Error bars represent 95 % confidence intervals. Target antigens are indicated on the x axis. Immunogenicity was assessed 1 month after receipt of the third of three doses at 2, 4, and 6 months of age co-administered with routine vaccines, at 12 months of age (pre-boost) before administration of the fourth (booster) dose co-administered with the measles-mumps-rubella vaccine, and 1 month (post-boost) after the booster vaccination. fHbp factor H-binding protein, hSBA human serum bactericidal assay, NadA Neisseria adhesin A, NHBA Neisseria heparin-binding antigen, PorA-OMV NZ porin A as part of the New Zealand strain outer membrane vesicles
Fig. 3
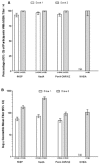
Immunogenicity in response to two doses of 4CMenB given 1 month apart in adolescents. a Percentage of participants with hSBA titer ≥4 at 1 month after each of two doses of 4CMenB administered 1 month apart to adolescents aged 11–17 years. b Geometric mean titers. Error bars represent 95 % confidence intervals. Target antigens are indicated on the x axis. fHbp factor H-binding protein, hSBA human serum bactericidal assay, NA not available, NadA Neisseria adhesin A, NHBA Neisseria heparin-binding antigen, PorA-OMV NZ porin A as part of the New Zealand strain outer membrane vesicles
Fig. 4
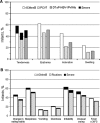
4CMenB tolerability in infants. a Solicited local reactions after dose 1. Injection site data are provided for 624 infants given 4CMenB, PCV7, and DTaP-HBV-IPV/Hib concomitantly. Erythema, swelling, and induration were characterized as severe if local reaction was >50 mm. Tenderness was categorized as severe if subject cried when injected limb was moved. b Solicited general reactions after dose 1. Reactogenicity rates are given for infants who received 4CMenB and routine vaccines separately, 4CMenB at 2, 4, 6 months and routines at 3, 5, 7 months. Data are shown for the first dose of 4CMenB and routines (at 2 and 3 months, respectively). Reactions were categorized as severe if subject was unable to perform normal daily activities; fever was severe if temperature was ≥40 °C. Routine vaccines were DTaP-HBV-IPV/Hib (combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio, and Haemophilus influenzae type b vaccine) and PCV7 (7-valent pneumococcal glycoconjugate vaccine)
Fig. 5
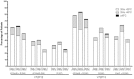
Fever rates for 4CMenB with concomitant routine vaccines vs. routine vaccines alone by dose in infants. Fever rates are given for infants who received 4CMenB, routine vaccines, or routines + MenC. Numbers in parentheses indicate the months in which the infant received the vaccine. For study V72P12, axillary temperatures were measured in 63–64 % of subjects and rectal temperatures in 35–37 % of subjects. For study V72P13, fever was measured rectally in almost all subjects. R routine vaccines, namely DTaP-HBV-IPV/Hib (combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio, and Haemophilus influenzae type b vaccine) and PCV7 (7-valent pneumococcal glycoconjugate vaccine)
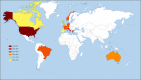
Fig. 6
Predicted capsular group B strain coverage of 4CMenB globally. The color code indicates the level of predicted coverage (no color indicates no MATS data available). MATS meningococcal antigen typing system
Similar articles
- Immune responses to a recombinant, four-component, meningococcal serogroup B vaccine (4CMenB) in adolescents: a phase III, randomized, multicentre, lot-to-lot consistency study.
Perrett KP, McVernon J, Richmond PC, Marshall H, Nissen M, August A, Percell S, Toneatto D, Nolan T. Perrett KP, et al. Vaccine. 2015 Sep 22;33(39):5217-24. doi: 10.1016/j.vaccine.2015.06.103. Epub 2015 Jul 29. Vaccine. 2015. PMID: 26232542 Clinical Trial. - Multicomponent meningococcal serogroup B vaccine (4CMenB; Bexsero(®)): a review of its use in primary and booster vaccination.
Carter NJ. Carter NJ. BioDrugs. 2013 Jun;27(3):263-74. doi: 10.1007/s40259-013-0029-2. BioDrugs. 2013. PMID: 23575646 Review. - Antibody persistence and booster response in adolescents and young adults 4 and 7.5 years after immunization with 4CMenB vaccine.
Nolan T, Santolaya ME, de Looze F, Marshall H, Richmond P, Henein S, Rheault P, Heaton K, Perrett KP, Garfield H, Gupta A, Ferguson M, D'Agostino D, Toneatto D, O'Ryan M. Nolan T, et al. Vaccine. 2019 Feb 21;37(9):1209-1218. doi: 10.1016/j.vaccine.2018.12.059. Epub 2019 Jan 26. Vaccine. 2019. PMID: 30691980 Clinical Trial. - MATS: Global coverage estimates for 4CMenB, a novel multicomponent meningococcal B vaccine.
Medini D, Stella M, Wassil J. Medini D, et al. Vaccine. 2015 May 28;33(23):2629-36. doi: 10.1016/j.vaccine.2015.04.015. Epub 2015 Apr 13. Vaccine. 2015. PMID: 25882169 Review. - Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study.
Santolaya ME, O'Ryan ML, Valenzuela MT, Prado V, Vergara R, Muñoz A, Toneatto D, Graña G, Wang H, Clemens R, Dull PM; V72P10 Meningococcal B Adolescent Vaccine Study group. Santolaya ME, et al. Lancet. 2012 Feb 18;379(9816):617-24. doi: 10.1016/S0140-6736(11)61713-3. Epub 2012 Jan 18. Lancet. 2012. PMID: 22260988 Clinical Trial.
Cited by
- Immunogenicity of a Meningococcal B Vaccine during a University Outbreak.
Basta NE, Mahmoud AA, Wolfson J, Ploss A, Heller BL, Hanna S, Johnsen P, Izzo R, Grenfell BT, Findlow J, Bai X, Borrow R. Basta NE, et al. N Engl J Med. 2016 Jul 21;375(3):220-8. doi: 10.1056/NEJMoa1514866. N Engl J Med. 2016. PMID: 27468058 Free PMC article. - 4CMenB journey to the 10-year anniversary and beyond.
Abitbol V, Martinón-Torres F, Taha MK, Nolan T, Muzzi A, Bambini S, Borrow R, Toneatto D, Serino L, Rappuoli R, Pizza M. Abitbol V, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2357924. doi: 10.1080/21645515.2024.2357924. Epub 2024 Jul 8. Hum Vaccin Immunother. 2024. PMID: 38976659 Free PMC article. Review. - Structures of NHBA elucidate a broadly conserved epitope identified by a vaccine induced antibody.
Maritan M, Veggi D, Cozzi R, Dello Iacono L, Bartolini E, Lo Surdo P, Maruggi G, Spraggon G, Bottomley MJ, Malito E. Maritan M, et al. PLoS One. 2018 Aug 22;13(8):e0201922. doi: 10.1371/journal.pone.0201922. eCollection 2018. PLoS One. 2018. PMID: 30133484 Free PMC article. - Role of Gonococcal Neisserial Surface Protein A (NspA) in Serum Resistance and Comparison of Its Factor H Binding Properties with Those of Its Meningococcal Counterpart.
Lewis LA, Rice PA, Ram S. Lewis LA, et al. Infect Immun. 2019 Jan 24;87(2):e00658-18. doi: 10.1128/IAI.00658-18. Print 2019 Feb. Infect Immun. 2019. PMID: 30510105 Free PMC article. - Screening and characterization of hypothetical proteins of Plasmodium falciparum as novel vaccine candidates in the fight against malaria using reverse vaccinology.
Aguttu C, Okech BA, Mukisa A, Lubega GW. Aguttu C, et al. J Genet Eng Biotechnol. 2021 Jul 16;19(1):103. doi: 10.1186/s43141-021-00199-y. J Genet Eng Biotechnol. 2021. PMID: 34269931 Free PMC article.
References
- European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe (2007); 2012. http://www.ecdc.europa.eu/en/publications/Publications/101011_SUR_Survei... (Accessed 14 Aug 2012).
- Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, Neisseria meningitidis; 2008. http://www.cdc.gov/abcs/reports-findings/survreports/mening08.html (Accessed 14 Aug 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical