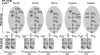Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems - PubMed (original) (raw)
Review
Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems
John C Kraft et al. J Pharm Sci. 2014 Jan.
Abstract
Liposomes are spherical-enclosed membrane vesicles mainly constructed with lipids. Lipid nanoparticles are loaded with therapeutics and may not contain an enclosed bilayer. The majority of those clinically approved have diameters of 50-300 nm. The growing interest in nanomedicine has fueled lipid-drug and lipid-protein studies, which provide a foundation for developing lipid particles that improve drug potency and reduce off-target effects. Integrating advances in lipid membrane research has enabled therapeutic development. At present, about 600 clinical trials involve lipid particle drug delivery systems. Greater understanding of pharmacokinetics, biodistribution, and disposition of lipid-drug particles facilitated particle surface hydration technology (with polyethylene glycol) to reduce rapid clearance and provide sufficient blood circulation time for drug to reach target tissues and cells. Surface hydration enabled the liposome-encapsulated cancer drug doxorubicin (Doxil) to gain clinical approval in 1995. Fifteen lipidic therapeutics are now clinically approved. Although much research involves attaching lipid particles to ligands selective for occult cells and tissues, preparation procedures are often complex and pose scale-up challenges. With emerging knowledge in drug target and lipid-drug distribution in the body, a systems approach that integrates knowledge to design and scale lipid-drug particles may further advance translation of these systems to improve therapeutic safety and efficacy.
Keywords: disposition; drug delivery systems; lipids; liposomes; micelle; nanoparticles; nanotechnology; pegylation; phospholipids.
© 2013 Wiley Periodicals, Inc. and the American Pharmacists Association.
Figures
Figure 1
A schematic presentation of commonly used phospholipids. Most of the commonly used lipids are presented with hydrophobic R1 and R2 fatty acyl tail groups and a hydrophilic head group carrying a net charge at neutral pH 7. The head group determines the charge of a phospholipid, whereas the lipid tail group contributes no charge. The lipids with head groups (oval shape shaded area) for sphingomyelin (SPH), phosphatidylcholine, and phosphatidylethanolamine exhibit neutral net charge. Phosphatidylserine and phosphatidylglycerol carry a negative net charge at neutral pH 7. The tail groups (R1 and R2) for each phospholipid can have various lengths (typically C14–C18) and degrees of saturation. SPH contains a sphingoid base backbone (unshaded) and the other four phospholipids contain a glycerol backbone (unshaded). In addition, R1 of SPH is a C15-saturated carbon chain and R2 is a fatty acid residue connected to the sphingoid base backbone through an amide functional group. The fatty acid residues for the other four phospholipids are attached to the glycerol backbone via an ester functional group. The detailed effects on the physical properties of phospholipids because of charge and variation in R1 and R2 are described in Table 3.
Figure 2
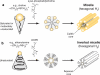
A schematic presentation of lipids and derivatives that form micelles and inverted micelles. (a) Lipidic micelles (hexagonal HI) are formed because of a large hydrophilic head group, such as a lyso-phosphatidylcholine with a choline head group and a saturated fatty acid. They form stable molecular aggregates that resemble sheets of tubes with an internal lipidic core. (b) Inverted micelles (hexagonal HII) are formed because of phospholipid with a neutral and small head group, such as phosphatidylethanolamine, with unsaturated fatty acyl tails that tend to form inverted cone structures in solution (e.g., 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine or DOPE). They form stable molecular aggregates that resemble sheets of tubes with an internal aqueous space.
Figure 3
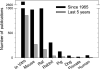
Number of publications on liposome research in vitro and in specific animal species. Data recorded in the PubMed database were identified with the search terms “liposome AND (‘_in vitro_’ or specific animal species).” For “human,” the term “clinical trial” was used for the search query. Data were compiled and plotted as a bar graph for the number of publications since 1965 and the last 5 years (2008–2013). A summary of numerical data is presented in Table 5.
Figure 4
Some common chemistry for conjugating targeting ligands to lipid anchors. (a) For short peptides containing about 3–20 amino acids, a 16-carbon chain palmitoyl or other fatty acid chains may be attached to the protein through a lysine, cysteine (Cys), or glycine residue on either the N-terminal or C-terminal end of the protein sequence. (b) A sulfhydryl-containing terminal Cys on a targeting peptide ligand can be coupled to succinimidyl 3-(2-pyridyldithio)propionate-activated phosphatidylcholine (PC) lipid anchors, forming disulfide bond linkages. (c) A sulfhydryl-containing terminal Cys on a targeting peptide ligand can be coupled to succinimidyl-4-(p-maleimidophenyl) butyrate-activated PC lipid anchors, forming stable thioether linkages. (d) An activated amino group on a targeting peptide ligand (e.g., transferrin) can be coupled to an activated carboxyl group on a PC lipid anchor [e.g., N-glutaryl phosphatidylethanolamine (NGPE)-PC] through a carbodiimide reaction with ethyl(dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS), forming peptide bond linkages. Addition of NHS to EDC reactions increases efficiency. The black dot indicates atoms that form the bond of interest.
Similar articles
- Constant pressure-controlled extrusion method for the preparation of Nano-sized lipid vesicles.
Morton LA, Saludes JP, Yin H. Morton LA, et al. J Vis Exp. 2012 Jun 22;(64):4151. doi: 10.3791/4151. J Vis Exp. 2012. PMID: 22760481 Free PMC article. - Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives.
Mukherjee A, Waters AK, Kalyan P, Achrol AS, Kesari S, Yenugonda VM. Mukherjee A, et al. Int J Nanomedicine. 2019 Mar 19;14:1937-1952. doi: 10.2147/IJN.S198353. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30936695 Free PMC article. Review. - Liposomal drug delivery systems: from concept to clinical applications.
Allen TM, Cullis PR. Allen TM, et al. Adv Drug Deliv Rev. 2013 Jan;65(1):36-48. doi: 10.1016/j.addr.2012.09.037. Epub 2012 Oct 1. Adv Drug Deliv Rev. 2013. PMID: 23036225 Review. - Trends and developments in liposome drug delivery systems.
Lian T, Ho RJ. Lian T, et al. J Pharm Sci. 2001 Jun;90(6):667-80. doi: 10.1002/jps.1023. J Pharm Sci. 2001. PMID: 11357170 - Nanoparticles containing insoluble drug for cancer therapy.
Guo S, Huang L. Guo S, et al. Biotechnol Adv. 2014 Jul-Aug;32(4):778-88. doi: 10.1016/j.biotechadv.2013.10.002. Epub 2013 Oct 8. Biotechnol Adv. 2014. PMID: 24113214 Free PMC article. Review.
Cited by
- From lab to industrial development of lipid nanocarriers using quality by design approach.
Buya AB, Mahlangu P, Witika BA. Buya AB, et al. Int J Pharm X. 2024 Jul 1;8:100266. doi: 10.1016/j.ijpx.2024.100266. eCollection 2024 Dec. Int J Pharm X. 2024. PMID: 39050378 Free PMC article. Review. - Nanoparticles as Drug Delivery Vehicles for People with Cystic Fibrosis.
Hourihane E, Hixon KR. Hourihane E, et al. Biomimetics (Basel). 2024 Sep 22;9(9):574. doi: 10.3390/biomimetics9090574. Biomimetics (Basel). 2024. PMID: 39329596 Free PMC article. Review. - Identification of an ALK-2 inhibitor as an agonist for intercellular exchange and tumor delivery of nanomaterial.
Wu X, Guo H, Zhao J, Wei Y, Li YX, Pang HB. Wu X, et al. Adv Ther (Weinh). 2023 Feb;6(2):2200173. doi: 10.1002/adtp.202200173. Epub 2022 Oct 17. Adv Ther (Weinh). 2023. PMID: 36818419 Free PMC article. - Transdermal Drug Delivery Systems and Their Use in Obesity Treatment.
Li Z, Fang X, Yu D. Li Z, et al. Int J Mol Sci. 2021 Nov 25;22(23):12754. doi: 10.3390/ijms222312754. Int J Mol Sci. 2021. PMID: 34884558 Free PMC article. Review. - Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR. Danaei M, et al. Pharmaceutics. 2018 May 18;10(2):57. doi: 10.3390/pharmaceutics10020057. Pharmaceutics. 2018. PMID: 29783687 Free PMC article. Review.
References
- Saunders L, Thomas IL. Diffusion studies with lysolecithin. J Chem Soc. 1958:483–485.
- Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–668. - PubMed
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. - PubMed
- Kinman L, Brodie SJ, Tsai CC, Bui T, Larsen K, Schmidt A, Anderson D, Morton WR, Hu SL, Ho RJ. Lipid–drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: A proof of concept study in HIV-2287-infected macaques. J Acquir Immune Defic Syndr. 2003;34(4):387–397. - PubMed
- James JS. DOXIL approved for KS. AIDS treatment news (no. 236) 1995:6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 HD083091/HD/NICHD NIH HHS/United States
- UL1TR000423/TR/NCATS NIH HHS/United States
- AI077390/AI/NIAID NIH HHS/United States
- P51 RR000166/RR/NCRR NIH HHS/United States
- R33 AI071971/AI/NIAID NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- RR00166/RR/NCRR NIH HHS/United States
- RR025014/RR/NCRR NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- AI071971/AI/NIAID NIH HHS/United States
- R21 AI071971/AI/NIAID NIH HHS/United States
- R01 AI077390/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources