Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines - PubMed (original) (raw)
Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines
Sabine U Vorrink et al. Toxicol Appl Pharmacol. 2014.
Abstract
The aryl hydrocarbon receptor (AhR) is an important mediator of toxic responses after exposure to xenobiotics including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and dioxin-like polychlorinated biphenyls (PCBs). Activation of AhR responsive genes requires AhR dimerization with the aryl hydrocarbon receptor nuclear translocator (ARNT), a heterodimeric partner also shared by the hypoxia-inducible factor-1α (HIF-1α) protein. TCDD-stimulated AhR transcriptional activity can be influenced by hypoxia; however, it less well known whether hypoxia interferes with AhR transcriptional transactivation in the context of PCB-mediated AhR activation in human cells. Elucidation of this interaction is important in liver hepatocytes which extensively metabolize ingested PCBs and experience varying degrees of oxygen tension during normal physiologic function. This study was designed to assess the effect of hypoxia on AhR transcriptional responses after exposure to 3,3',4,4',5-pentachlorobiphenyl (PCB 126). Exposure to 1% O2 prior to PCB 126 treatment significantly inhibited CYP1A1 mRNA and protein expression in human HepG2 and HaCaT cells. CYP1A1 transcriptional activation was significantly decreased upon PCB 126 stimulation under conditions of hypoxia. Additionally, hypoxia pre-treatment reduced PCB 126 induced AhR binding to CYP1 target gene promoters. Importantly, ARNT overexpression rescued cells from the inhibitory effect of hypoxia on XRE-luciferase reporter activity. Therefore, the mechanism of interference of the signaling crosstalk between the AhR and hypoxia pathways appears to be at least in part dependent on ARNT availability. Our results show that AhR activation and CYP1A1 expression induced by PCB 126 were significantly inhibited by hypoxia and hypoxia might therefore play an important role in PCB metabolism and toxicity.
Keywords: 2,2′,4,4′,5,5′-hexachlorobiphenyl; 2,3,7,8-tetrachlorodibenzo-p-dioxin; 3,3′,4,4′,5-pentachlorobiphenyl; 6,2′,4′-trimethoxyflavone; ARNT; AhR; CYP1A1; ChIP; DMSO; EMSA; HIF-1α; HRE; Hypoxia; PCB; PCB 126; PCB 153; RPLP0; TCDD; TMF; XRE; aryl hydrocarbon receptor; aryl hydrocarbon receptor nuclear translocator; bHLH/PAS; basic helix-loop-helix/PER-ARNT-SIM; chromatin immunoprecipitation; cytochrome P450 1A1; dimethyl sulfoxide; electrophoretic mobility shift assay; hypoxia response element; hypoxia-inducible factor-1α; polychlorinated biphenyl; qRT-PCR; quantitative real-time reverse transcription polymerase chain reaction; ribosomal protein, large, P0; xenobiotic response element.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
The authors declare that no conflicts of interest exist.
Figures
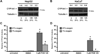
Figure 1. Oxygen concentration dependent induction of CYP1A1 mRNA expression
HepG2 cells (left panel: A-C) and HaCaT cells (right panel: D-F) were incubated in normoxia (21% O2) or hypoxia (1% O2) for 8 hours prior to treatment with vehicle (DMSO), 2–4 nM PCB 126, 0.1–3 µM PCB 126 or 3 µM TCDD for 6 hours or 4 hours, respectively. CYP1A1 mRNA expression was determined by qRT-PCR. Analysis was performed with normalization to RPLP0 mRNA. (A, D) Dose-response curves for PCB 126 treated cells in normal oxygen and hypoxia. The EC20 is indicated by a solid black line in normoxic cells and a dashed line in hypoxic cells. (B, C and E, F) CYP1A1 mRNA expression levels of untreated and treated cells including standard error. RNA expression levels significantly (p < 0.05) greater than untreated controls are indicated by an asterisk (*). RNA expression levels significantly (p < 0.05) different between the PCB 126 treated samples in normoxia and hypoxia are indicated by a number sign (#). Error bars = SEM. n = 4 for PCB 126, n = 3 for TCDD.
Figure 2. CYP1A1 protein levels and enzyme activity are reduced in low oxygen environments
Upper panel: HepG2 cells (A) and HaCaT cells (B) were subjected to normoxia or hypoxia for 16 hours prior to treatment with 3 µM PCB 126 for 48 hours or 24 hours, respecitively. Ten micrograms of whole cell lysate were western blotted for the presence of CYP1A1. Beta-tubulin was used as a loading control. Numbers over the lanes represent relative intensities of CYP1A1 compared to PCB 126 treated cells in normoxia. n.d. = not detectable, n.s. = non-specific band. Lower panel: HepG2 cells (C) and HaCaT cells (D) were incubated in normoxia or hypoxia for 16 hours before treatment with 3 µM PCB 126 for 24 hours. CYP1A1 activity was measured using a luminogenic CYP1A1 substrate and is depicted relative to untreated cells in normoxia. * = p < 0.005 vs. control. # = p < 0.005 normoxia vs. hypoxia. Error bars = SEM. n = 3.
Figure 3. Hypoxia inhibits PCB 126 induced CYP1A1 promoter-luciferase reporter activity
HepG2 cells (A) and HaCaT cells (B) were transfected with a XRE-luciferase (firefly) reporter vector and Renilla luciferase vector and then subjected to normoxia or hypoxia for 16 hours prior to treatment with 3 µM PCB 126 for 4 or 6 hours. Firefly luminescence was determined and normalized to Renilla luminescence, and is depicted relative to untreated cells in normoxia. * = p < 0.005 vs. control. # = p < 0.005 normoxia vs. hypoxia. Error bars = SEM. n = 3.
Figure 4. ARNT overexpression relieves hypoxic inhibition of CYP1A1 promoter-luciferase reporter activity
HepG2 cells were transfected with a XRE-luciferase (firefly) reporter vector, Renilla luciferase vector, and ARNT expression vector or empty control vector. Transfected cells were subjected to normoxia or hypoxia for 16 hours prior to treatment with 3 µM PCB 126 for 6 hours. Firefly luminescence was determined and is depicted relative to untreated cells in normoxia in the empty control vector group. * = p < 0.05 vs. control. # = p < 0.05 normoxia vs. hypoxia. ‡ = p < 0.05 empty vector vs. ARNT expression vector. Error bars = SEM. n = 3.
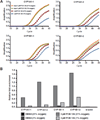
Figure 5. Differential binding of the AhR to CYP1A1 and CYP1B1 promoters after PCB 126 treatment
HaCaT cells were subjected to normoxia or hypoxia for 16 hours and subsequently treated with 3 µM PCB 126 for 1 hour. ChIP assays were performed using an anti-AhR antibody. Enriched DNA was analyzed using qRT-PCR primers designed to regions in the promoters of CYP1A1 and CYP1B1. Enrichment at the beta-actin promoter was used as a negative control. (A) Magnitude of the signal (delta Rn) vs. cycle number. (B) Enrichment levels calculated by subtracting CT values of ChIP DNA from input DNA in each sample.
Similar articles
- NcoA2-Dependent Inhibition of HIF-1α Activation Is Regulated via AhR.
Tsai CH, Li CH, Liao PL, Cheng YW, Lin CH, Huang SH, Kang JJ. Tsai CH, et al. Toxicol Sci. 2015 Dec;148(2):517-30. doi: 10.1093/toxsci/kfv199. Epub 2015 Sep 8. Toxicol Sci. 2015. PMID: 26350169 - Novel roles for AhR and ARNT in the regulation of alcohol dehydrogenases in human hepatic cells.
Attignon EA, Leblanc AF, Le-Grand B, Duval C, Aggerbeck M, Rouach H, Blanc EB. Attignon EA, et al. Arch Toxicol. 2017 Jan;91(1):313-324. doi: 10.1007/s00204-016-1700-4. Epub 2016 Apr 8. Arch Toxicol. 2017. PMID: 27055685 - Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex.
Beischlag TV, Wang S, Rose DW, Torchia J, Reisz-Porszasz S, Muhammad K, Nelson WE, Probst MR, Rosenfeld MG, Hankinson O. Beischlag TV, et al. Mol Cell Biol. 2002 Jun;22(12):4319-33. doi: 10.1128/MCB.22.12.4319-4333.2002. Mol Cell Biol. 2002. PMID: 12024042 Free PMC article. - Functional role of AhR in the expression of toxic effects by TCDD.
Mimura J, Fujii-Kuriyama Y. Mimura J, et al. Biochim Biophys Acta. 2003 Feb 17;1619(3):263-8. doi: 10.1016/s0304-4165(02)00485-3. Biochim Biophys Acta. 2003. PMID: 12573486 Review. - Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node.
Vorrink SU, Domann FE. Vorrink SU, et al. Chem Biol Interact. 2014 Jul 25;218:82-8. doi: 10.1016/j.cbi.2014.05.001. Epub 2014 May 10. Chem Biol Interact. 2014. PMID: 24824450 Free PMC article. Review.
Cited by
- Effects of Naphtho[2,1-a]pyrene Exposure on CYP1A1 Expression: An in Vivo and in Vitro Mechanistic Study Exploring the Role of m6A Posttranscriptional Modification.
Shen J, Wang L, Zhang W, Gong X, Li S, Zou X, Chen C, Xia R, Zhang D, Xu S, Xu J, Wang S, Jiang Y, Sun H, Wang C, Wang SL. Shen J, et al. Environ Health Perspect. 2024 Aug;132(8):87003. doi: 10.1289/EHP14055. Epub 2024 Aug 12. Environ Health Perspect. 2024. PMID: 39133094 Free PMC article. - Magnetophoretic circuits: A review of device designs and implementation for precise single-cell manipulation.
Abedini-Nassab R, Sadeghidelouei N, Shields Iv CW. Abedini-Nassab R, et al. Anal Chim Acta. 2023 Sep 1;1272:341425. doi: 10.1016/j.aca.2023.341425. Epub 2023 May 31. Anal Chim Acta. 2023. PMID: 37355317 Free PMC article. Review. - A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes.
Szelest M, Walczak K, Plech T. Szelest M, et al. Int J Mol Sci. 2021 Jan 22;22(3):1104. doi: 10.3390/ijms22031104. Int J Mol Sci. 2021. PMID: 33499346 Free PMC article. Review. - Back to the future: transgenerational transmission of xenobiotic-induced epigenetic remodeling.
Jiménez-Chillarón JC, Nijland MJ, Ascensão AA, Sardão VA, Magalhães J, Hitchler MJ, Domann FE, Oliveira PJ. Jiménez-Chillarón JC, et al. Epigenetics. 2015;10(4):259-73. doi: 10.1080/15592294.2015.1020267. Epub 2015 Mar 16. Epigenetics. 2015. PMID: 25774863 Free PMC article. Review. - Integrated transcriptomics and metabolomics reveal signatures of lipid metabolism dysregulation in HepaRG liver cells exposed to PCB 126.
Mesnage R, Biserni M, Balu S, Frainay C, Poupin N, Jourdan F, Wozniak E, Xenakis T, Mein CA, Antoniou MN. Mesnage R, et al. Arch Toxicol. 2018 Aug;92(8):2533-2547. doi: 10.1007/s00204-018-2235-7. Epub 2018 Jun 14. Arch Toxicol. 2018. PMID: 29947894 Free PMC article.
References
- Chubb LS, Andersen ME, Broccardo CJ, Legare ME, Billings RE, Dean CE, Hanneman WH. Regional induction of CYP1A1 in rat liver following treatment with mixtures of PCB 126 and PCB 153. Toxicol. Pathol. 2004;32:467–473. - PubMed
- Crinnion WJ. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Altern. Med. Rev. 2011;16:5–13. - PubMed
- Hansen LG, DeCaprio AP, Nisbet ICT. PCB congener comparisons reveal exposure histories for residents of Anniston, Alabama, USA. Fresenius Environ. Bull. 2003;12:181–190.
Publication types
MeSH terms
Substances
Grants and funding
- T32 ES007091/ES/NIEHS NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- P30 ES006694/ES/NIEHS NIH HHS/United States
- R01 CA115438/CA/NCI NIH HHS/United States
- P42 ES013661-5099/ES/NIEHS NIH HHS/United States
- P42 ES004940/ES/NIEHS NIH HHS/United States
- P42 ES004940-5083/ES/NIEHS NIH HHS/United States
- 2T32 ES007091-31/ES/NIEHS NIH HHS/United States
- P42 ES013661/ES/NIEHS NIH HHS/United States
- P50ES006694/ES/NIEHS NIH HHS/United States
- P42ES013661-5110/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources