Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study - PubMed (original) (raw)
Randomized Controlled Trial
Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study
John M Lachin et al. Diabetes Care. 2014.
Abstract
OBJECTIVE To describe the beneficial long-term effects of an average of 6.5 years of intensive diabetes therapy (INT) in type 1 diabetes on measures of atherosclerosis, cardiac structure and function, and clinical cardiovascular events observed in the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) study. RESEARCH DESIGN AND METHODS The DCCT was a randomized clinical trial of 1,441 participants assigned to receive INT or conventional therapy (CON). It was conducted between 1983-1993 with an average follow-up of 6.5 years. EDIC (1994-present) is an observational follow-up of the DCCT cohort. Cardiovascular events have been recorded throughout. During EDIC common carotid intima-media thickness (IMT) was measured with ultrasound, coronary artery calcification with computed tomography, and cardiac structure and function with cardiac magnetic resonance imaging. RESULTS DCCT INT and lower levels of HbA1c during DCCT/EDIC were associated with thinner carotid IMT, less coronary calcification, and a lower incidence of clinical cardiovascular events including myocardial infarction, stroke, and cardiac death. While there were no significant differences in cardiac structure and function between the former INT and CON groups, they were significantly associated with higher HbA1c during DCCT/EDIC. CONCLUSIONS DCCT INT and the attendant 6.5 years of lower HbA1c had long-term salutary effects on the development and progression of atherosclerosis and cardiovascular disease during the subsequent follow-up during EDIC.
Figures
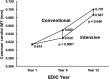
Figure 1
The mean level of the common carotid IMT within the INT and CON groups at EDIC year 1 and again at EDIC years 6 and 12. Reprinted with permission from Polak et al. (12).
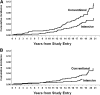
Figure 2
The cumulative incidence of clinical CVD outcomes during DCCT/EDIC. A: Any qualifying primary outcome event. B: MACE. Reprinted with permission from Nathan et al. (9).
Similar articles
- The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study.
Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, Zinman B, Jacobson A, Sun W, Lachin JM, Nathan DM; DCCT/EDIC Research Group. Cleary PA, et al. Diabetes. 2006 Dec;55(12):3556-65. doi: 10.2337/db06-0653. Diabetes. 2006. PMID: 17130504 Free PMC article. Clinical Trial. - Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study.
de Boer IH; DCCT/EDIC Research Group. de Boer IH, et al. Diabetes Care. 2014;37(1):24-30. doi: 10.2337/dc13-2113. Diabetes Care. 2014. PMID: 24356594 Free PMC article. Clinical Trial. - Realising the long-term promise of insulin therapy: the DCCT/EDIC study.
Nathan DM. Nathan DM. Diabetologia. 2021 May;64(5):1049-1058. doi: 10.1007/s00125-021-05397-4. Epub 2021 Feb 6. Diabetologia. 2021. PMID: 33550441 Review.
Cited by
- Management of Patients with Newly Diagnosed Diabetic Mellitus: Ophthalmologic Outcomes in Intensive versus Conventional Glycemic Control.
Lam PY, Chow SC, Lam WC, Chow LLW, Fung NSK. Lam PY, et al. Clin Ophthalmol. 2021 Jun 25;15:2767-2785. doi: 10.2147/OPTH.S301317. eCollection 2021. Clin Ophthalmol. 2021. PMID: 34234400 Free PMC article. Review. - Exploring Racial and Ethnic Differences in Arterial Stiffness Among Youth and Young Adults With Type 1 Diabetes.
Sauder KA, Glueck DH, Harrall KK, D'Agostino R Jr, Dolan LM, Lane AD, Liese AD, Lustigova E, Malik FS, Marcovina S, Mayer-Davis E, Mottl A, Pihoker C, Reynolds K, Shah AS, Urbina EM, Wagenknecht LE, Daniels SR, Dabelea D. Sauder KA, et al. J Am Heart Assoc. 2023 Apr 4;12(7):e028529. doi: 10.1161/JAHA.122.028529. Epub 2023 Mar 30. J Am Heart Assoc. 2023. PMID: 36994741 Free PMC article. - Association Between Type 1 Diabetes Mellitus and Celiac Disease: Autoimmune Disorders With a Shared Genetic Background.
Flores Monar GV, Islam H, Puttagunta SM, Islam R, Kundu S, Jha SB, Rivera AP, Sange I. Flores Monar GV, et al. Cureus. 2022 Mar 7;14(3):e22912. doi: 10.7759/cureus.22912. eCollection 2022 Mar. Cureus. 2022. PMID: 35399440 Free PMC article. Review. - A comparison of IDeg + IAsp versus IDet + IAsp in subjects with type 1 diabetes: subgroup analysis of Japanese subjects.
Ono Y, Nishida T, Hyllested-Winge J, Seino H, Sasaki T. Ono Y, et al. Diabetol Int. 2016 Apr 5;7(4):404-412. doi: 10.1007/s13340-016-0267-x. eCollection 2016 Dec. Diabetol Int. 2016. PMID: 30603293 Free PMC article. - Cost-effectiveness analysis of a pharmacotherapeutic empowerment strategy for patients with type 2 diabetes mellitus.
Gonçalves ACO, Cazarim MS, Sanches C, Pereira LRL, Camargos AMT, Aquino JA, Oliveira Baldoni A. Gonçalves ACO, et al. BMJ Open Diabetes Res Care. 2019 Jul 16;7(1):e000647. doi: 10.1136/bmjdrc-2018-000647. eCollection 2019. BMJ Open Diabetes Res Care. 2019. PMID: 31413839 Free PMC article.
References
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
- Lachin J, Genuth S, Nathan D, Davis M, The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 - PMC - PubMed
- Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes 2008;57:995–1001 - PubMed
- Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 - PMC - PubMed
- Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK094176/DK/NIDDK NIH HHS/United States
- U01-DK-094157/DK/NIDDK NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- U01 DK094157/DK/NIDDK NIH HHS/United States
- U01-DK-094176/DK/NIDDK NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- UL1 TR000114/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical