Embedded multicellular spheroids as a biomimetic 3D cancer model for evaluating drug and drug-device combinations - PubMed (original) (raw)
Embedded multicellular spheroids as a biomimetic 3D cancer model for evaluating drug and drug-device combinations
Kristie M Charoen et al. Biomaterials. 2014 Feb.
Abstract
Multicellular aggregates of cells, termed spheroids, are of interest for studying tumor behavior and for evaluating the response of pharmacologically active agents. Spheroids more faithfully reproduce the tumor macrostructure found in vivo compared to classical 2D monolayers. We present a method for embedding spheroids within collagen gels followed by quantitative and qualitative whole spheroid and single cell analyses enabling characterization over the length scales from molecular to macroscopic. Spheroid producing and embedding capabilities are demonstrated for U2OS and MDA-MB-231 cell lines, of osteosarcoma and breast adenocarcinoma origin, respectively. Finally, using the MDA-MB-231 tumor model, the chemotherapeutic response between paclitaxel delivery as a bolus dose, as practiced in the clinic, is compared to delivery within an expansile nanoparticle. The expansile nanoparticle delivery route provides a superior outcome and the results mirror those observed in a murine xenograft model. These findings highlight the synergistic beneficial results that may arise from the use of a drug delivery system, and the need to evaluate both drug candidates and delivery systems in the research and preclinical screening phases of a new cancer therapy development program.
Keywords: 3D cell culture; Cancer model; Cell migration; Collagen; Drug delivery; Spheroid; Tumor mimic.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
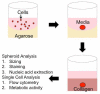
Figure 1
Creation of Embedded Spheroids: Spheroid formation is encouraged by placing a suspension of cells (red) in media (pink) on agarose (yellow) coated wells. After 72 hours, a spheroid is formed, and then transferred into a collagen gel.
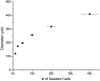
Figure 2
Controllable Spheroid Size: Spheroid size can be controlled by varying cell seeding number. Sizing based on cell seeding number was demonstrated with both bone (top) and breast (bottom) cancer cells.
Figure 2
Controllable Spheroid Size: Spheroid size can be controlled by varying cell seeding number. Sizing based on cell seeding number was demonstrated with both bone (top) and breast (bottom) cancer cells.
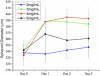
Figure 3
U2OS Spheroid Growth in Collagen: Bone cancer spheroids grown in collagen gels with varying mechanical properties demonstrated the most growth in 3-4 mg/mL, growing less in both stiffer and weaker gels.
Figure 3
U2OS Spheroid Growth in Collagen: Bone cancer spheroids grown in collagen gels with varying mechanical properties demonstrated the most growth in 3-4 mg/mL, growing less in both stiffer and weaker gels.
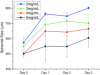
Figure 4
MDA-MB 231 Spheroid Growth in Collagen: Breast cancer spheroids grown in collagen gels with varying mechanical properties demonstrated the most growth in the weakest collagen gels.
Figure 4
MDA-MB 231 Spheroid Growth in Collagen: Breast cancer spheroids grown in collagen gels with varying mechanical properties demonstrated the most growth in the weakest collagen gels.
Figure 5
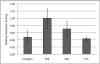
Pattern of Metabolic Activity: A spheroid in collagen was stained with a calcein based stain (green) to show metabolically active cells and an ethidium based stain (red) to demonstrate cells with a compromised membrane. The red staining on the interior indicates a mostly dead core, whereas the green cells shows a metabolically active outer ring of cells. The inset quantitatively demonstrates that 85.5% of the spheroid is alive after 3 days via FACS.
Figure 6
Metabolic Activity of Spheroids: Disaggregated spheroids were plated in a monolayer and the metabolic activity was compared to cells grown only in a monolayer. Although there were approximately 60,000 cells, the overall metabolic level is comparable to 30,000 cells.
Figure 7
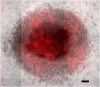
Nanoparticle Penetration: Within 24 hours fluorescently labeled nanoparticles were able to fully penetrate a 20,000 cell MDA-MB 231 spheroid. Scale bar is 100 _μ_m.
Figure 8
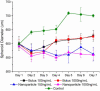
Spheroid Treatment with Nanoparticles: Paclitaxel, a chemotherapeutic drug was delivered via bolus dose or nanoparticles for 24 hours before the treatment was removed. The nanoparticle delivery method was the most effective at reducing spheroid size.
Similar articles
- Real-time viability and apoptosis kinetic detection method of 3D multicellular tumor spheroids using the Celigo Image Cytometer.
Kessel S, Cribbes S, Bonasu S, Rice W, Qiu J, Chan LL. Kessel S, et al. Cytometry A. 2017 Sep;91(9):883-892. doi: 10.1002/cyto.a.23143. Epub 2017 Jun 15. Cytometry A. 2017. PMID: 28618188 - Optimization of the formation of embedded multicellular spheroids of MCF-7 cells: How to reliably produce a biomimetic 3D model.
Zhang W, Li C, Baguley BC, Zhou F, Zhou W, Shaw JP, Wang Z, Wu Z, Liu J. Zhang W, et al. Anal Biochem. 2016 Dec 15;515:47-54. doi: 10.1016/j.ab.2016.10.004. Epub 2016 Oct 5. Anal Biochem. 2016. PMID: 27717854 - A 3D biomimetic model of tissue stiffness interface for cancer drug testing.
Lam CR, Wong HK, Nai S, Chua CK, Tan NS, Tan LP. Lam CR, et al. Mol Pharm. 2014 Jul 7;11(7):2016-21. doi: 10.1021/mp500059q. Epub 2014 Apr 22. Mol Pharm. 2014. PMID: 24754837 - Applicability of tumor spheroids for in vitro chemosensitivity assays.
Hamilton G, Rath B. Hamilton G, et al. Expert Opin Drug Metab Toxicol. 2019 Jan;15(1):15-23. doi: 10.1080/17425255.2019.1554055. Epub 2018 Dec 2. Expert Opin Drug Metab Toxicol. 2019. PMID: 30484335 Review. - Anticancer drug discovery using multicellular tumor spheroid models.
Zanoni M, Pignatta S, Arienti C, Bonafè M, Tesei A. Zanoni M, et al. Expert Opin Drug Discov. 2019 Mar;14(3):289-301. doi: 10.1080/17460441.2019.1570129. Epub 2019 Jan 28. Expert Opin Drug Discov. 2019. PMID: 30689452 Review.
Cited by
- Cancer cell spheroids are a better screen for the photodynamic efficiency of glycosylated photosensitizers.
Pereira PMR, Berisha N, Bhupathiraju NVSDK, Fernandes R, Tomé JPC, Drain CM. Pereira PMR, et al. PLoS One. 2017 May 17;12(5):e0177737. doi: 10.1371/journal.pone.0177737. eCollection 2017. PLoS One. 2017. PMID: 28545086 Free PMC article. - Establishment of a 3D Co-culture With MDA-MB-231 Breast Cancer Cell Line and Patient-Derived Immune Cells for Application in the Development of Immunotherapies.
Saraiva DP, Matias AT, Braga S, Jacinto A, Cabral MG. Saraiva DP, et al. Front Oncol. 2020 Aug 27;10:1543. doi: 10.3389/fonc.2020.01543. eCollection 2020. Front Oncol. 2020. PMID: 32974189 Free PMC article. - 3D Biomimetic Models to Reconstitute Tumor Microenvironment In Vitro: Spheroids, Organoids, and Tumor-on-a-Chip.
Li W, Zhou Z, Zhou X, Khoo BL, Gunawan R, Chin YR, Zhang L, Yi C, Guan X, Yang M. Li W, et al. Adv Healthc Mater. 2023 Jul;12(18):e2202609. doi: 10.1002/adhm.202202609. Epub 2023 Mar 29. Adv Healthc Mater. 2023. PMID: 36917657 Free PMC article. Review. - Construction of a Fibroblast-Associated Tumor Spheroid Model Based on a Collagen Drop Array Chip.
Roh H, Kim H, Park JK. Roh H, et al. Biosensors (Basel). 2021 Dec 9;11(12):506. doi: 10.3390/bios11120506. Biosensors (Basel). 2021. PMID: 34940263 Free PMC article. - Hydrogels to model 3D in vitro microenvironment of tumor vascularization.
Song HH, Park KM, Gerecht S. Song HH, et al. Adv Drug Deliv Rev. 2014 Dec 15;79-80:19-29. doi: 10.1016/j.addr.2014.06.002. Epub 2014 Jun 23. Adv Drug Deliv Rev. 2014. PMID: 24969477 Free PMC article. Review.
References
- Hutmacher DW. Biomaterials offer cancer research the third dimension. Nature Mater. 2010;9:90–3. - PubMed
- Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro - a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15(5):405–12. - PubMed
- Dhiman HK, Ray AR, Panda AK. Characterization and evaluation of chitosan matrix for in vitro growth of MCF-7 breast cancer cell lines. Biomaterials. 2004;25(21):5147–54. - PubMed
- Dhiman HK, Ray AR, Panda AK. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials. 2005;26(9):979–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA149561/CA/NCI NIH HHS/United States
- T32 EB006359/EB/NIBIB NIH HHS/United States
- T32EB006359/EB/NIBIB NIH HHS/United States
- T32 GM008541/GM/NIGMS NIH HHS/United States
- R25 CA153955/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous