Danger signals in the initiation of the inflammatory response after myocardial infarction - PubMed (original) (raw)
Review
Danger signals in the initiation of the inflammatory response after myocardial infarction
J J de Haan et al. Mediators Inflamm. 2013.
Abstract
During myocardial infarction, sterile inflammation occurs. The danger model is a solid theoretic framework that explains this inflammation as danger associated molecular patterns activate the immune system. The innate immune system can sense danger signals through different pathogen recognition receptors (PRR) such as toll-like receptors, nod-like receptors and receptors for advanced glycation endproducts. Activation of a PRR results in the production of cytokines and the recruitment of leukocytes to the site of injury. Due to tissue damage and necrosis of cardiac cells, danger signals such as extracellular matrix (ECM) breakdown products, mitochondrial DNA, heat shock proteins and high mobility box 1 are released. Matricellular proteins are non-structural proteins expressed in the ECM and are upregulated upon injury. Some members of the matricellular protein family (like tenascin-C, osteopontin, CCN1 and the galectins) have been implicated in the inflammatory and reparative responses following myocardial infarction and may function as danger signals. In a clinical setting, danger signals can function as prognostic and/or diagnostic biomarkers and for drug targeting. In this review we will provide an overview of the established knowledge on the role of danger signals in myocardial infarction and we will discuss areas of interest for future research.
Figures
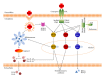
Figure 1
DAMP signaling through different PRRs. TLR activation by DAMPS triggers adaptor proteins MyD88, TRIF, TIRAP, or TRAM to activate various transcriptions factors. The subsequent translocation of NF-κ_B and MAPK leads to the production of several proinflammatory cytokines. TRIF-dependent activation of transcription factors IRF3 and IRF7 results in the induction of type I interferon. Additionally, the TLR- NF-κ_B pathway can induce the transcription of pro-IL-1_β, pro-IL-18, and other components of the inflammasome pathway. Inflammasome activation is considered to depend on two distinct signals. The first signal via TLR and this might be the rate limiting step for inflammasome assembly and activity; the second signal via NLR which is responsible for inflammasome assembly, caspase-1 activation, and secretion of IL-1_β and IL-18. Activation of NOD receptors results in activation of the NF-_κ_B pathway.
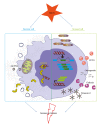
Figure 2
Proposed simplified mediators of danger signal release during myocardial infarction. Necrotic cells in the myocardium are leaky and release a subset of DAMPs, for instance, mtDNA and the DNA binding protein HMGB1. Furthermore, viable cells get stress signals from their surroundings and start to produce and secrete a range of proteins. These cells start the production of the EDA splice variant of fibronectin, HSPs and the matricellular proteins CCN1, osteopontin, galectins, and tenascin-C. Both the proteins released by necrotic cells and the produced proteins by stressed cells are able to activate or aggravate the immune response in the heart following MI.
Similar articles
- HMGB1: endogenous danger signaling.
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. Klune JR, et al. Mol Med. 2008 Jul-Aug;14(7-8):476-84. doi: 10.2119/2008-00034.Klune. Mol Med. 2008. PMID: 18431461 Free PMC article. Review. - The impact of endogenous triggers on trauma-associated inflammation.
Zedler S, Faist E. Zedler S, et al. Curr Opin Crit Care. 2006 Dec;12(6):595-601. doi: 10.1097/MCC.0b013e3280106806. Curr Opin Crit Care. 2006. PMID: 17077693 Review. - Innate immunity and cardiomyocytes in ischemic heart disease.
Lin L, Knowlton AA. Lin L, et al. Life Sci. 2014 Mar 28;100(1):1-8. doi: 10.1016/j.lfs.2014.01.062. Epub 2014 Jan 28. Life Sci. 2014. PMID: 24486305 Free PMC article. Review. - Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1).
van Beijnum JR, Buurman WA, Griffioen AW. van Beijnum JR, et al. Angiogenesis. 2008;11(1):91-9. doi: 10.1007/s10456-008-9093-5. Epub 2008 Feb 9. Angiogenesis. 2008. PMID: 18264787 Review. - The role of HMGB1/RAGE in inflammatory cardiomyopathy.
Volz HC, Kaya Z, Katus HA, Andrassy M. Volz HC, et al. Semin Thromb Hemost. 2010 Mar;36(2):185-94. doi: 10.1055/s-0030-1251503. Epub 2010 Apr 22. Semin Thromb Hemost. 2010. PMID: 20414834 Review.
Cited by
- Fibrosis after Myocardial Infarction: An Overview on Cellular Processes, Molecular Pathways, Clinical Evaluation and Prognostic Value.
Scalise RFM, De Sarro R, Caracciolo A, Lauro R, Squadrito F, Carerj S, Bitto A, Micari A, Bella GD, Costa F, Irrera N. Scalise RFM, et al. Med Sci (Basel). 2021 Mar 1;9(1):16. doi: 10.3390/medsci9010016. Med Sci (Basel). 2021. PMID: 33804308 Free PMC article. Review. - The danger model approach to the pathogenesis of the rheumatic diseases.
Pacheco-Tena C, González-Chávez SA. Pacheco-Tena C, et al. J Immunol Res. 2015;2015:506089. doi: 10.1155/2015/506089. Epub 2015 Apr 20. J Immunol Res. 2015. PMID: 25973436 Free PMC article. Review. - CaMKIIδ-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis.
Willeford A, Suetomi T, Nickle A, Hoffman HM, Miyamoto S, Heller Brown J. Willeford A, et al. JCI Insight. 2018 Jun 21;3(12):e97054. doi: 10.1172/jci.insight.97054. eCollection 2018 Jun 21. JCI Insight. 2018. PMID: 29925681 Free PMC article. - Regulation of Mitochondrial Quality Control by Natural Drugs in the Treatment of Cardiovascular Diseases: Potential and Advantages.
Chang X, Zhang W, Zhao Z, Ma C, Zhang T, Meng Q, Yan P, Zhang L, Zhao Y. Chang X, et al. Front Cell Dev Biol. 2020 Dec 23;8:616139. doi: 10.3389/fcell.2020.616139. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33425924 Free PMC article. Review. - Modulation of Macrophage Polarization and HMGB1-TLR2/TLR4 Cascade Plays a Crucial Role for Cardiac Remodeling in Senescence-Accelerated Prone Mice.
Karuppagounder V, Giridharan VV, Arumugam S, Sreedhar R, Palaniyandi SS, Krishnamurthy P, Quevedo J, Watanabe K, Konishi T, Thandavarayan RA. Karuppagounder V, et al. PLoS One. 2016 Apr 12;11(4):e0152922. doi: 10.1371/journal.pone.0152922. eCollection 2016. PLoS One. 2016. PMID: 27070323 Free PMC article.
References
- Matzinger P. Tolerance, danger, and the extended family. Annual Review of Immunology. 1994;12:991–1045. - PubMed
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Current Opinion in Immunology. 2001;13(1):114–119. - PubMed
- Chun K-H, Seong S-Y. CD14 but not MD2 transmit signals from DAMP. International Immunopharmacology. 2010;10(1):98–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials