A hereditary spastic paraplegia mouse model supports a role of ZFYVE26/SPASTIZIN for the endolysosomal system - PubMed (original) (raw)
doi: 10.1371/journal.pgen.1003988. Epub 2013 Dec 19.
Katrin Kollmann 2, Nicole Koch 3, Christoph Biskup 4, Sandor Nietzsche 5, Geraldine Zimmer 1, J Christopher Hennings 1, Antje K Huebner 1, Judit Symmank 1, Amir Jahic 6, Elena I Ilina 7, Kathrin Karle 8, Ludger Schöls 8, Michael Kessels 3, Thomas Braulke 2, Britta Qualmann 3, Ingo Kurth 1, Christian Beetz 6, Christian A Hübner 1
Affiliations
- PMID: 24367272
- PMCID: PMC3868532
- DOI: 10.1371/journal.pgen.1003988
A hereditary spastic paraplegia mouse model supports a role of ZFYVE26/SPASTIZIN for the endolysosomal system
Mukhran Khundadze et al. PLoS Genet. 2013.
Abstract
Hereditary spastic paraplegias (HSPs) are characterized by progressive weakness and spasticity of the legs because of the degeneration of cortical motoneuron axons. SPG15 is a recessively inherited HSP variant caused by mutations in the ZFYVE26 gene and is additionally characterized by cerebellar ataxia, mental decline, and progressive thinning of the corpus callosum. ZFYVE26 encodes the FYVE domain-containing protein ZFYVE26/SPASTIZIN, which has been suggested to be associated with the newly discovered adaptor protein 5 (AP5) complex. We show that Zfyve26 is broadly expressed in neurons, associates with intracellular vesicles immunopositive for the early endosomal marker EEA1, and co-fractionates with a component of the AP5 complex. As the function of ZFYVE26 in neurons was largely unknown, we disrupted Zfyve26 in mice. Zfyve26 knockout mice do not show developmental defects but develop late-onset spastic paraplegia with cerebellar ataxia confirming that SPG15 is caused by ZFYVE26 deficiency. The morphological analysis reveals axon degeneration and progressive loss of both cortical motoneurons and Purkinje cells in the cerebellum. Importantly, neuron loss is preceded by accumulation of large intraneuronal deposits of membrane-surrounded material, which co-stains with the lysosomal marker Lamp1. A density gradient analysis of brain lysates shows an increase of Lamp1-positive membrane compartments with higher densities in Zfyve26 knockout mice. Increased levels of lysosomal enzymes in brains of aged knockout mice further support an alteration of the lysosomal compartment upon disruption of Zfyve26. We propose that SPG15 is caused by an endolysosomal membrane trafficking defect, which results in endolysosomal dysfunction. This appears to be particularly relevant in neurons with highly specialized neurites such as cortical motoneurons and Purkinje cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
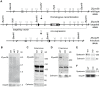
Figure 1. Zfyve26 is highly expressed in neuronal cells.
(A) According to the Western blot analysis (80 µg protein per lane) using a novel anti-C-terminus antibody Zfyve26 is expressed in different tissues. (B,C) In situ hybridization of sagittal sections of a brain from a 2-month-old wild-type mouse with a Zfyve26 sense-control probe (B) or the _Zfyve26_-specific antisense probe (C). Higher magnification of labeled areas in (C): (C′) olfactory bulb, (C″) motor cortex, (C′″) hippocampus, (C″″) cerebellar cortex (Purkinje cells are indicated by arrows). (D,D′) In situ hybridization of transversal spinal cord sections. Lower motoneurons in the anterior horn of the spinal cord are labeled as well. Scale bars: 500 µm (B,C), 50 µm (C′–C′″,D), 100 µm (C″″,D′). GCL: granule cell layer, GL: glomeruli, MC: mitral cells, PCL: Purkinje cell layer, PG: periglomerular cells, ML: molecular layer.
Figure 2. Targeted disruption of the murine Zfyve26 gene.
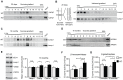
(A) Genomic structure of the Zfyve26 gene (top) and the targeted Zfyve26 locus (middle). The dotted line indicates the extent of the targeting construct. A neomycin cassette (Neo) flanked by frt sites (black boxes) and a loxP-site (black triangle) was inserted into intron 15. A second loxP-site together with a _Bam_HI site was introduced into intron 14. Correctly targeted ES cell clones were selected for the generation of chimeric mice. Zfyve26 knockout mice were established by breeding chimeric mice to a cre-deleter mouse strain to obtain constitutive Zfyve26 knockout mice. (B) Northern blot analysis of total brain RNA from wild-type (WT), Zfyve26 heterozygous (HET), and Zfyve26 knockout (KO) animals. Gapdh served as loading control. (C,D) Western blot analysis with affinity purified antibodies against the N-terminus (C) or the C-terminus (D) of Zfyve26 detected a 285 kD Zfyve26 polypeptide in brain extracts from wild-type but not from knockout mice. Calnexin served as a loading control. (E) Spatacsin levels, an interaction partner of Zfyve26, are reduced in brain lysates of Zfyve26 knockout mice. Calnexin was used as a loading control. (F) Spatacsin is not regulated on the transcriptional level in Zfyve26 knockout mice. Gapdh was used as a loading control.
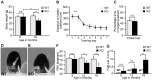
Figure 3. Zfyve26 knockout mice develop a progressive spastic and ataxic gait disorder.
(A) Whereas the bodyweight did not differ between genotypes at 8 months of age, it was reduced for knockout mice at 16 months of age (KO: n = 8; WT: n = 8; **: p<0.001). (B–C) At 5 months of age no obvious learning and memory deficits were noted in the Morris water maze (KO: n = 15; WT: n = 14; p>0.05). (D–E) Single video frames of a wild-type and knockout mouse walking on a beam. The foot-base-angle (FBA) at toe off position is indicated by white lines. (F) In contrast to WT mice (n = 10) the FBA decreased with age in Zfyve26 knockout mice (n = 10). (G) Zfyve26 knockout mice (n = 10) fell from the beam more often (**: p<0.001) compared to WT littermates (n = 10). Error bars represent SEM. Statistical analysis of repeated behavioral tests and body weight measurements were analyzed with 2-way ANOVA followed by a Bonferroni test. **: p<0.001, ***: p<0.0001. n.s.: not significant.
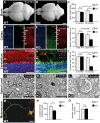
Figure 4. Disruption of Zfyve26 causes severe neuron loss in the motor cortex and cerebellum.
(A–B) The brain was smaller in 16-month-old Zfyve26 knockout compared to wild-type mice. Scale bars: 2 mm. (C) Progressive reduction of brain weight in Zfyve26 knockout mice (n = 4; 2-way ANOVA; **: p<0.001). (D,E) Astrogliosis and loss of NeuN-positive neuronal cells in the motor cortex of 16-month-old Zfyve26 knockout mice. Hoechst-33258 (blue; nuclei), GFAP (green; astrocyte marker), and NeuN (red; neuronal marker) staining of the motor cortex at 16 months of age from wild-type (D) and knockout (E) mice. Individual cortical layers are labeled (I–VI). Scale bars: 100 µm. (F) Quantification of NeuN-positive cells per layer revealed a significant reduction of neurons from layers V–VI of the motor cortex in 16-month-old Zfyve26 knockout mice (Student's t-test; **: p<0.001). (G–H) Cerebellar sections stained for GFAP (green), Calbindin (red, Purkinje cell marker), and Hoechst-33258 (blue) revealed a severe loss of Purkinje cells in 16-month-old knockout mice. Scale bars: 100 µm. GCL: granule cell layer, PCL: Purkinje cell layer, ML: molecular layer. (I) In knockout mice Purkinje cells were drastically reduced at 16 months (2-way ANOVA; ***: p<0.0001), but not at 2 months of age. (J–K) Semithin sections of the lumbar corticospinal tract illustrates the reduction in the number of large diameter axons in 16-month-old knockout mice. Scale bars: 20 µm. (L) Transmission electron microscopy of a degenerating axon in a Zfyve26 knockout mouse. Scale bar: 1 µm. (M,N) Delayed outgrowth of Tau-positive axons of cultured motoneurons isolated from knockout (KO) embryos compared to wild-type (WT) mice. (Student's t-test; **: p<0.001). Scale bar in M: 40 µm. (O) The number of axonal branches in cultured motoneurons did not differ between genotypes. Error bars represent SEM. (Student's t-test; n.s.: not significant).
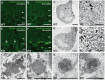
Figure 5. Accumulation of autofluorescent, electron-dense, membrane-enclosed material in knockout mice.
(A,E) In knockout mice autofluorescent material (excited at 488 nm) was already observed in neurons at 2 months of age (white arrows). (B,F) At 16 months of age these deposits were drastically enlarged in knockout mice. Purkinje cell somata in (A–B,E–F) are indicated by a dashed line. ML: molecular layer; PCL: Purkinje cell layer; GCL: granule cell layer. Scale bars: 25 µm. (C–D,G–H) Although lipofuscin particles were found in both wild-type and knockout mice at 16 months of age, abnormal clusters of membrane enclosed deposits were only observed in knockout samples (H, highlighted region). For clarity the tissue surrounding the Purkinje cell soma has been dampened in (C) and (G). The magnification of the highlighted regions in (C) and (G) are shown in (D) and (H). A regular lipofuscin particle in (D) and (H) is marked with an black arrow. The borders of the abnormal cluster shown in (H) are indicated with arrowheads. (I) Large membrane enclosed deposits were also found in KO Purkinje cells. Regular lysosomal vesicles were noted both in wild-type (J) and knockout samples (K). (L) In knockout samples some lysosomal structures showed similarities with fingerprint bodies, which are typical for juvenile neuronal ceroid lipofuscinosis. Scale bars: 5 µm (C,G), 2 µm (D,H,I) and 0.5 µm (J–L).
Figure 6. Large autofluorescent particles in knockout tissues are Lamp1-positive.
Confocal microscopy of cerebellar sections of 10-month-old mice. (A,B,C,D) Maximum intensity projections of all channels analyzed. (A–B′″) Autofluorescent deposits (green) in Purkinje cells barely co-localized with EEA1 (red). (C–D′″) Autofluorescent deposits in knockout tissues were Lamp1-positive (clone CD107a). Scale bars: 10 µm.
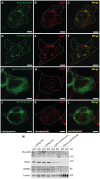
Figure 7. Zfyve26 is associated with endolysosomal membranes.
(A–C) A ZFYVE26-GFP fusion protein expressed in 3T3 cells was associated with vesicular structures positive for the early endosomal marker protein EEA1. (D–F) There was only a partial association between ZFYVE26-GFP and the late endosomal/lysosomal marker protein Lamp1. (G–I) The vesicular staining was absent in cells transfected with the ZFYVE26-GFP variant harboring the point mutation His1834Ala in the FYVE-domain. (J–L) Pretreatment with wortmannin, an inhibitor of phosphatidylinositol-3-kinases, interfered with the vesicular staining of the FYVE-domain proteins ZFYVE26 and EEA1. Scale bars: 15 µm. (M) Subcellular fractionation of brain homogenates of WT and Zfyve26 knockout mice followed by immunoblotting for Zfyve26, its interaction partner AP5M1/μ5, and the marker proteins EEA1 and Lamp1 showed that both Zfyve26 and the AP5 complex were found in the light membrane fraction. Heavy membranes fractions enriched for Lamp1 lacked EEA1, AP5M1/μ5, and Zfyve26-reactive polypeptides.
Figure 8. In brains of aged knockout mice lysosomal compartments show abnormal densities and lysosomal enzyme activities are increased.
(A,B) Density gradient analyses of Lamp1-positive membrane isolations from 20-day-old wild-type and Zfyve26 knockout mice with 2 different protocols revealed an increased presence of Lamp1-positive membrane compartments with higher density in Zfyve26 knockout material compared to wild-type. (C) A density shift was also observed at 16 months of age (same fractionation protocol as displayed in A). (D) No shift was detected for the AP5M1/μ5 subunit. (E) Quantitative Western blot analysis of Lamp1, Cathepsin D (CtsDm: mature; CtsDp: precursor), or EEA1 in 1.000 g supernatants from brain lysates of 16-month-old mice. Only Cathepsin D levels were significantly increased (n = 5; Student's t-test; *: p<0.05). (F,G) In brain lysates of Zfyve26 knockout mice both β-hexosaminidase (F) and β-galactosidase (G) activity was increased at 16 months, but not at 2 months of age (n = 3; 2-way ANOVA; *: p<0.01).
Similar articles
- Axon demyelination and degeneration in a zebrafish spastizin model of hereditary spastic paraplegia.
Garg V, André S, Heyer L, Kracht G, Ruhwedel T, Scholz P, Ischebeck T, Werner HB, Dullin C, Engelmann J, Möbius W, Göpfert MC, Dosch R, Geurten BRH. Garg V, et al. Open Biol. 2024 Nov;14(11):240100. doi: 10.1098/rsob.240100. Epub 2024 Nov 6. Open Biol. 2024. PMID: 39503232 Free PMC article. - ZFYVE26/SPASTIZIN and SPG11/SPATACSIN mutations in hereditary spastic paraplegia types AR-SPG15 and AR-SPG11 have different effects on autophagy and endocytosis.
Vantaggiato C, Panzeri E, Castelli M, Citterio A, Arnoldi A, Santorelli FM, Liguori R, Scarlato M, Musumeci O, Toscano A, Clementi E, Bassi MT. Vantaggiato C, et al. Autophagy. 2019 Jan;15(1):34-57. doi: 10.1080/15548627.2018.1507438. Epub 2018 Sep 13. Autophagy. 2019. PMID: 30081747 Free PMC article. - Defective autophagy in spastizin mutated patients with hereditary spastic paraparesis type 15.
Vantaggiato C, Crimella C, Airoldi G, Polishchuk R, Bonato S, Brighina E, Scarlato M, Musumeci O, Toscano A, Martinuzzi A, Santorelli FM, Ballabio A, Bresolin N, Clementi E, Bassi MT. Vantaggiato C, et al. Brain. 2013 Oct;136(Pt 10):3119-39. doi: 10.1093/brain/awt227. Epub 2013 Sep 11. Brain. 2013. PMID: 24030950 Free PMC article. - Current Knowledge of Endolysosomal and Autophagy Defects in Hereditary Spastic Paraplegia.
Toupenet Marchesi L, Leblanc M, Stevanin G. Toupenet Marchesi L, et al. Cells. 2021 Jul 2;10(7):1678. doi: 10.3390/cells10071678. Cells. 2021. PMID: 34359848 Free PMC article. Review. - Ataxia and spastic paraplegia in mitochondrial disease.
Synofzik M, Rugarli E, Reid E, Schüle R. Synofzik M, et al. Handb Clin Neurol. 2023;194:79-98. doi: 10.1016/B978-0-12-821751-1.00009-9. Handb Clin Neurol. 2023. PMID: 36813322 Review.
Cited by
- Update on the Genetics of Spastic Paraplegias.
Boutry M, Morais S, Stevanin G. Boutry M, et al. Curr Neurol Neurosci Rep. 2019 Feb 28;19(4):18. doi: 10.1007/s11910-019-0930-2. Curr Neurol Neurosci Rep. 2019. PMID: 30820684 Review. - Towards a better understanding of the neuro-developmental role of autophagy in sickness and in health.
Zapata-Muñoz J, Villarejo-Zori B, Largo-Barrientos P, Boya P. Zapata-Muñoz J, et al. Cell Stress. 2021 Jun 29;5(7):99-118. doi: 10.15698/cst2021.07.253. eCollection 2021 Jul. Cell Stress. 2021. PMID: 34308255 Free PMC article. Review. - Lysosome Function and Dysfunction in Hereditary Spastic Paraplegias.
Edmison D, Wang L, Gowrishankar S. Edmison D, et al. Brain Sci. 2021 Jan 24;11(2):152. doi: 10.3390/brainsci11020152. Brain Sci. 2021. PMID: 33498913 Free PMC article. Review. - Axon demyelination and degeneration in a zebrafish spastizin model of hereditary spastic paraplegia.
Garg V, André S, Heyer L, Kracht G, Ruhwedel T, Scholz P, Ischebeck T, Werner HB, Dullin C, Engelmann J, Möbius W, Göpfert MC, Dosch R, Geurten BRH. Garg V, et al. Open Biol. 2024 Nov;14(11):240100. doi: 10.1098/rsob.240100. Epub 2024 Nov 6. Open Biol. 2024. PMID: 39503232 Free PMC article. - Antibodies to the RNA-binding protein hnRNP A1 contribute to neurodegeneration in a model of central nervous system autoimmune inflammatory disease.
Douglas JN, Gardner LA, Salapa HE, Lalor SJ, Lee S, Segal BM, Sawchenko PE, Levin MC. Douglas JN, et al. J Neuroinflammation. 2016 Jul 8;13(1):178. doi: 10.1186/s12974-016-0647-y. J Neuroinflammation. 2016. PMID: 27391474 Free PMC article.
References
- Depienne C, Stevanin G, Brice A, Durr A (2007) Hereditary spastic paraplegias: an update. Curr Opin Neurol 20: 674–680. - PubMed
- Harding AE (1983) Classification of the hereditary ataxias and paraplegias. Lancet 1: 1151–1155. - PubMed
- Reid E (1999) The hereditary spastic paraplegias. J Neurol 246: 995–1003. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
The study was supported by grants of the DFG DE 807/8-1 (CAH), QU116/5-2 (BQ), KE685/3-1 (MKe), the IZKF Jena, and the Thyssenfoundation to CAH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous