New insights in extracellular matrix remodeling and collagen turnover related pathways in cultured human tenocytes after ciprofloxacin administration - PubMed (original) (raw)
. 2013 Aug 11;3(3):122-31.
eCollection 2013 Jul.
Affiliations
- PMID: 24367771
- PMCID: PMC3838320
New insights in extracellular matrix remodeling and collagen turnover related pathways in cultured human tenocytes after ciprofloxacin administration
Alessandra Menon et al. Muscles Ligaments Tendons J. 2013.
Abstract
We characterized the effect of ciprofloxacin (CPX) in cultured human tenocytes by morphological and molecular methods. Collagen type I and III mRNA and protein levels were unaffected, but lysyl hydroxylase 2b mRNA levels progressively decreased after CPX administration. MMP-1 protein levels significantly increased after 20 μg/ml CPX administration but remained unmodified at the higher dose, whilst MMP-2 activity was unchanged. Tissue inhibitor of MMP (TIMP-1) gene expression decreased after CPX treatment, whilst TIMP-2 and transforming growth factor-β1 gene expression, the cytoskeleton arrangement, and cytochrome c expression remained unmodified. Secreted Protein Acidic and Rich in Cysteine mRNA and protein levels remained almost unchanged, whilst N-cadherin mRNA levels resulted significantly down-regulated and connexin 43 gene expression tended to decrease after CPX administration. The CPX-induced decreased ability to cross-link collagen and decreased TIMP-1 levels, possibly leading to higher activity of MMPs in ECM degradation, together with the down-regulation of N-cadherin and connexin 43 are consistent with a reduced ability to maintain tissue homeostasis, possibly making the tendon more susceptible to rupture.
Keywords: ciprofloxacin; collagen turnover; extra-cellular matrix remodelling; tendons; tenocytes.
Figures
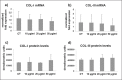
Figure 1.
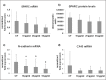
Bar graphs showing COL-I (a) and COL-III mRNA levels (b) assessed by real time PCR in untreated (CT) and CPX-treated tenocytes. Data were normalized on GAPDH gene expression. (c) Bar graphs displaying COL-I and COL-III (d) protein levels analyzed by slot blot in culture medium of CT and CPX treated tenocytes. Data are mean ± SD for two independent experiments for samples run in duplicate.
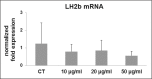
Figure 2.
Bar graphs showing mRNA levels for LH2b in CT and tenocytes treated with CPX at different doses as described in the Materials and methods section. Data were normalized on GAPDH gene expression and are expressed as mean ± SD for two independent experiments for samples run in duplicate.
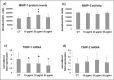
Figure 3.
(a) Bar graphs showing MMP-1 protein levels analyzed by slot blot and MMP-2 activity assessed by SDS-zymography (b) in tenocyte serum-free conditioned media after densitometric analysis of immunoreactive and lytic bands, respectively. Data are expressed as densitometric units ± SD for two independent experiments for samples run in duplicate. (c) Bar graphs showing TIMP-1 and TIMP-2 (d) gene expression after normalization on GAPDH mRNA levels. Data are expressed as mean ± SD for two independent experiments for samples run in duplicate. *p<0.01 vs CT; ^p<0.05 vs CT; °p<0.05 vs 10 _μ_g/ml
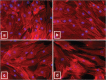
Figure 4.
Immunofluorescence analysis of the actin cytoskeleton in tenocytes untreated and treated with CPX. Representative immunofluorescence photomicrographs of microfilament distribution, evidenced by rhodamine-phalloidin labeling, in CT (a) and tenocytes after 10 _μ_g/ml (b), 20 _μ_g/ml (c) and 50 _μ_g/ml CPX (d). DAPI was used for nuclear staining. Original magnification: 40×.
Figure 5.
Representative immunofluorescence analysis for vimentin intermediate filaments in CT (a) and tenocytes after 10 _μ_g/ml (b), 20 _μ_g/ml (c) and 50 _μ_g/ml CPX (d). DAPI was used for nuclear staining. Original magnification: 40×.
Figure 6.
Representative immunofluorescence photomicrographs of microtubules in CT (a) and tenocytes after 10 _μ_g/ml (b), 20 _μ_g/ml (c) and 50 _μ_g/ml CPX (d). DAPI was used for nuclear staining. Original magnification: 40×.
Figure 7.
Bar graphs showing SPARC gene expression (a) and protein levels in cell culture super-natants (b), N-cadherin (c) and CX43 (d) mRNA levels in tenocytes untreated (CT) and after CPX administration. Data were normalized on GAPDH mRNA levels and are expressed as mean ± SD for two independent experiments for samples run in duplicate. *P<0.05 vs CT; °P<0.01 vs CT
Figure 8.
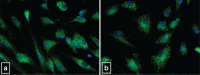
Intrinsic apoptosis analysis. Representative microphotographs showing immunofluorescence detection of cytochrome c in CT (a) and 50 _μ_g/ml CPX-treated (b) tenocytes. Untreated CT and CPX-treated cells showed a similar immunoreactivity characterized by a punctate cytoplasmic staining pattern typical for localization of cytochrome c into intact mitochondria. Original magnification 40×.
Similar articles
- Uremic Toxins and Ciprofloxacin Affect Human Tenocytes In Vitro.
Popowski E, Kohl B, Schneider T, Jankowski J, Schulze-Tanzil G. Popowski E, et al. Int J Mol Sci. 2020 Jun 14;21(12):4241. doi: 10.3390/ijms21124241. Int J Mol Sci. 2020. PMID: 32545914 Free PMC article. - Expression profiling of genes involved in collagen turnover in tendons from cerebral palsy patients.
Gagliano N, Pelillo F, Chiriva-Internati M, Picciolini O, Costa F, Schutt RC Jr, Gioia M, Portinaro N. Gagliano N, et al. Connect Tissue Res. 2009;50(3):203-8. doi: 10.1080/03008200802613630. Connect Tissue Res. 2009. PMID: 19444761 - Control of extracellular matrix homeostasis of normal cartilage by a TGFbeta autocrine pathway. Validation of flow cytometry as a tool to study chondrocyte metabolism in vitro.
Wang L, Almqvist KF, Veys EM, Verbruggen G. Wang L, et al. Osteoarthritis Cartilage. 2002 Mar;10(3):188-98. doi: 10.1053/joca.2001.0492. Osteoarthritis Cartilage. 2002. PMID: 11869079 - Effect of a Collagen-Based Compound on Morpho-Functional Properties of Cultured Human Tenocytes.
Randelli F, Menon A, Giai Via A, Mazzoleni MG, Sciancalepore F, Brioschi M, Gagliano N. Randelli F, et al. Cells. 2018 Dec 6;7(12):246. doi: 10.3390/cells7120246. Cells. 2018. PMID: 30563214 Free PMC article. - MMPS and TIMPS in ovarian physiology and pathophysiology.
Goldman S, Shalev E. Goldman S, et al. Front Biosci. 2004 Sep 1;9:2474-83. doi: 10.2741/1409. Front Biosci. 2004. PMID: 15353300 Review.
Cited by
- Short-Term Exposure to Ciprofloxacin Reduces Proteoglycan Loss in Tendon Explants.
James S, Daffy J, Cook J, Samiric T. James S, et al. Genes (Basel). 2022 Nov 25;13(12):2210. doi: 10.3390/genes13122210. Genes (Basel). 2022. PMID: 36553476 Free PMC article. - Effect of Ciprofloxacin on Susceptibility to Aortic Dissection and Rupture in Mice.
LeMaire SA, Zhang L, Luo W, Ren P, Azares AR, Wang Y, Zhang C, Coselli JS, Shen YH. LeMaire SA, et al. JAMA Surg. 2018 Sep 1;153(9):e181804. doi: 10.1001/jamasurg.2018.1804. Epub 2018 Sep 19. JAMA Surg. 2018. PMID: 30046809 Free PMC article. - A novel injectable fibromodulin-releasing granular hydrogel for tendon healing and functional recovery.
Xu X, Zhang Y, Ha P, Chen Y, Li C, Yen E, Bai Y, Chen R, Wu BM, Da Lio A, Ting K, Soo C, Zheng Z. Xu X, et al. Bioeng Transl Med. 2022 Jul 14;8(1):e10355. doi: 10.1002/btm2.10355. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684085 Free PMC article. - A Protocol to Acquire the Degenerative Tenocyte from Humans.
Han SH, Kim HK, Ahn JH, Lee DH, Baek M, Ye G, Lee JM, Min K, Oh C, Lee S. Han SH, et al. J Vis Exp. 2018 Jun 9;(136):57634. doi: 10.3791/57634. J Vis Exp. 2018. PMID: 29939181 Free PMC article. - Relationship between fluoroquinolones and the risk of aortic diseases: a meta-analysis of observational studies.
Dai XC, Yang XX, Ma L, Tang GM, Pan YY, Hu HL. Dai XC, et al. BMC Cardiovasc Disord. 2020 Feb 3;20(1):49. doi: 10.1186/s12872-020-01354-y. BMC Cardiovasc Disord. 2020. PMID: 32013928 Free PMC article.
References
- Pierfitte C, Royer RJ. Tendon disorders with fluoroquinolones. Therapie. 1996;51:419–420. - PubMed
- Khaliq Y, Zhanel GG. Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clin Infect Dis. 2003;36:1404–1410. - PubMed
- Ozaras R, Mert A, Tahan V, Uraz S, Ozaydin I, Yilmaz MH, Ozaras N. Ciprofloxacin and Achilles’ tendon rupture: a causal relationship. Clin Rheumatol. 2003;22:500–501. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous