mRNA cap methylation influences pathogenesis of vesicular stomatitis virus in vivo - PubMed (original) (raw)
mRNA cap methylation influences pathogenesis of vesicular stomatitis virus in vivo
Yuanmei Ma et al. J Virol. 2014 Mar.
Abstract
One role of mRNA cap guanine-N-7 (G-N-7) methylation is to facilitate the efficient translation of mRNA. The role of mRNA cap ribose 2'-O methylation is enigmatic, although recent work has implicated this as a signature to avoid detection of RNA by the innate immune system (S. Daffis, K. J. Szretter, J. Schriewer, J. Q. Li, S. Youn, J. Errett, T. Y. Lin, S. Schneller, R. Zust, H. P. Dong, V. Thiel, G. C. Sen, V. Fensterl, W. B. Klimstra, T. C. Pierson, R. M. Buller, M. Gale, P. Y. Shi, M. S. Diamond, Nature 468:452-456, 2010, doi:10.1038/nature09489). Working with vesicular stomatitis virus (VSV), we previously showed that a panel of recombinant VSVs carrying mutations at a predicted methyltransferase catalytic site (rVSV-K1651A, -D1762A, and -E1833Q) or S-adenosylmethionine (SAM) binding site (rVSV-G1670A, -G1672A, and -G4A) were defective in cap methylation and were also attenuated for growth in cell culture. Here, we analyzed the virulence of these recombinants in mice. We found that rVSV-K1651A, -D1762A, and -E1833Q, which are defective in both G-N-7 and 2'-O methylation, were highly attenuated in mice. All three viruses elicited a high level of neutralizing antibody and provided full protection against challenge with the virulent VSV. In contrast, mice inoculated with rVSV-G1670A and -G1672A, which are defective only in G-N-7 methylation, were attenuated in vivo yet retained a low level of virulence. rVSV-G4A, which is completely defective in both G-N-7 and 2'-O methylation, also exhibited low virulence in mice despite the fact that productive viral replication was not detected in lung and brain. Taken together, our results suggest that abrogation of viral mRNA cap methylation can serve as an approach to attenuate VSV, and perhaps other nonsegmented negative-strand RNA viruses, for potential application as vaccines and viral vectors.
Importance: Nonsegmented negative-sense (NNS) RNA viruses include a wide range of significant human, animal, and plant pathogens. For many of these viruses, there are no vaccines or antiviral drugs available. mRNA cap methylation is essential for mRNA stability and efficient translation. Our current understanding of mRNA modifications of NNS RNA viruses comes largely from studies of vesicular stomatitis virus (VSV). In this study, we showed that recombinant VSVs (rVSVs) defective in mRNA cap methylation were attenuated in vitro and in vivo. In addition, these methyltransferase (MTase)-defective rVSVs triggered high levels of antibody responses and provided complete protection against VSV infection. Thus, this study will not only contribute to our understanding of the role of mRNA cap MTase in viral pathogenesis but also facilitate the development of new live attenuated vaccines for VSV, and perhaps other NNS RNA viruses, by inhibiting viral mRNA cap methylation.
Figures
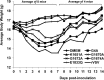
FIG 1
Dynamics of mouse body weight change after VSV infection. Four-week-old SPF female BALB/c mice (8 mice per group) were inoculated with each MTase-defective VSVs intranasally at a dose of 5×106 PFU. At day 7 postinoculation, 4 mice from each group were euthanized. The body weight of the remaining 4 mice in each group was monitored until day 14 postinoculation. The average body weight for each group is shown.
FIG 2
Histopathological changes in lungs after VSV infection. Four-week-old SPF female BALB/c mice were inoculated with each MTase-defective VSVs intranasally at a dose of 5 × 106 PFU. Mice from each group were euthanized at day 7 postinoculation. The right lung from each mouse was fixed in 4% (vol/vol) phosphate-buffered paraformaldehyde, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin (HE). Detailed criteria for evaluation of lung histological change are described in Materials and Methods. Representative lung histology from each group is shown. DMEM control, grade 0 (no lesion); rVSV, grade 3 (severe); rVSV-E1833Q, grade 1 (mild); rVSV-G1670A, grade 2 (moderate); and rVSV-G4A, grade 3 (severe).
FIG 3
Histopathological changes in brain after VSV infection. Mice were euthanized at day 7 postinoculation. A horizontal brain section containing the olfactory bulb, forebrain, cerebrum, hippocampus, and cerebellum was fixed in 4% (vol/vol) phosphate-buffered paraformaldehyde, followed by HE straining. Detailed criteria for evaluation of brain histological change are described in Materials and Methods. DMEM control, grade 0 (no lesion); rVSV-K1651A, grade 1 (mild); rVSV-G4A, grade 1 (mild); and rVSV, grade 3 (severe).
FIG 4
Detection of viral RNA in lung and brain by RT-PCR. Equal amounts (0.5 g) of lung and brain tissues were homogenized in 0.5 ml of DMEM. Total RNA was extracted from each sample, followed by RT-PCR to amplify the VSV N gene. The amplified products were analyzed on a 1% agarose gel. M indicates DNA marker; lanes 1 to 4 indicate the numbers of mice.
FIG 5
Phenotype of rVSV-G4A isolated from mice. (A) Plaque morphology of rVSV-G4A on Vero cells. rVSV-G4A-M, virus isolated from lungs of rVSV-G4A-infected mice; rVSV-M, virus isolated from lungs of rVSV-infected mice. Plaques of rVSV and rVSV-M were developed after 24 h; those of rVSV-G4A, and rVSV-G4A-M were developed after 96 h. (B) [3H]SAM incorporation monitored by scintillation counting. Viral RNA was synthesized in vitro by using 10 μg of purified virus. The amount of [3H]SAM incorporated into the RNA was determined by scintillation counting, and the number of dpm was normalized to the amount of RNA synthesized. Three independent experiments were used to generate the graph.
FIG 6
Quantification of IFN-β mRNA by real-time RT-PCR. Mice were infected with rVSV, rVSV-G4A, rVSV-K1651A, and rVSV-G1670A at dose of 5 × 106 PFU/mouse. At days 3 postinfection, lung and brain tissues were collected. Total RNA was extracted from lung or brain tissues and was treated with DNase I (Ambion). The IFN-β mRNA in each tissue was quantified by real-time RT-PCR. The average amounts of IFN-β mRNA from 5 mice for each group are shown.
FIG 7
Replication and methylation of VSV in different cell lines. (A) Replication of rVSV-G4A is cell type dependent. Confluent BHK-21, Vero, or HEp-2 cells were infected with rVSV or G4A at an MOI of 0.1. After 24 h, supernatants were harvested, and virus titers were determined by plaque assay. (B) rVSV-G4A is defective in G-N-7 methylation in virus-infected cells. Confluent BHK-21, Vero cells, or HEp-2 in 35-mm dishes were infected by rVSV or G4A at an MOI of 0.1. After 24 h postinfection, poly(A)-containing viral mRNA was isolated. Equal amounts of viral RNA were incubated with 10 units of vaccinia virus G-N-7 MTase supplied with the m7G capping system (ScriptCap) in the presence of 15 μCi [3H]SAM (85 Ci/mmol; PerkinElmer). The G-N-7 methylation was measured by 3H incorporation using a 1414 series scintillation counter (PerkinElmer). (C) rVSV-G4A is defective in 2′-O methylation in virus-infected cells. Poly(A)-containing viral mRNA was incubated with 10 units of vaccinia virus G-N-7 MTase in the presence of 100 μM cold SAM. RNA was purified and incubated with vaccinia virus 2′-O MTase (VP39) in the presence of 15 μCi [3H]SAM. The 2′-O methylation was measured by 3H incorporation using a 1414 series scintillation counter.
FIG 8
Dynamics of mouse body weight change after primary immunization followed by virulent challenge. Four-week-old SPF female BALB/c mice were immunized with MTase-defective VSVs intranasally at a dose of 5 × 106 PFU per mouse. At week 4 postimmunization, mice were challenged with rVSV at a dose of 5 × 106 PFU per mouse. The body weight for each mouse was monitored every 3 days. The average body weight of four mice is shown. DMEMC, inoculated with DMEM and challenged with rVSV.
FIG 9
MTase-defective VSVs triggered high levels of antibody responses in mice. Blood samples were collected from each mouse weekly, and serum was isolated for antibody detection by ELISA. Antibody titers were calculated by the geometric mean titers (GMT). (A) Antibody titer after primary immunization. (B) Antibody titer after booster immunization.
FIG 10
Dynamics of mouse body weight change after booster immunization followed by virulent challenge. Four-week-old SPF female BALB/c mice were immunized with MTase-defective VSVs intranasally at a dose of 5 × 106 PFU per mouse. At week 2 after primary immunization, mice were boosted intranasally with 5 × 106 PFU of the corresponding virus. At week 3 after booster immunization, mice were challenged with rVSV at a dose of 5 × 106 PFU per mouse. The body weight for each mouse was monitored every 3 days. The average body weight of four mice is shown.
Similar articles
- A Methyltransferase-Defective Vesicular Stomatitis Virus-Based SARS-CoV-2 Vaccine Candidate Provides Complete Protection against SARS-CoV-2 Infection in Hamsters.
Lu M, Zhang Y, Dravid P, Li A, Zeng C, Kc M, Trivedi S, Sharma H, Chaiwatpongsakorn S, Zani A, Kenney A, Cai C, Ye C, Liang X, Qiu J, Martinez-Sobrido L, Yount JS, Boyaka PN, Liu SL, Peeples ME, Kapoor A, Li J. Lu M, et al. J Virol. 2021 Sep 27;95(20):e0059221. doi: 10.1128/JVI.00592-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379509 Free PMC article. - Methylation of viral mRNA cap structures by PCIF1 attenuates the antiviral activity of interferon-β.
Tartell MA, Boulias K, Hoffmann GB, Bloyet LM, Greer EL, Whelan SPJ. Tartell MA, et al. Proc Natl Acad Sci U S A. 2021 Jul 20;118(29):e2025769118. doi: 10.1073/pnas.2025769118. Proc Natl Acad Sci U S A. 2021. PMID: 34266951 Free PMC article. - Vesicular stomatitis viruses resistant to the methylase inhibitor sinefungin upregulate RNA synthesis and reveal mutations that affect mRNA cap methylation.
Li J, Chorba JS, Whelan SP. Li J, et al. J Virol. 2007 Apr;81(8):4104-15. doi: 10.1128/JVI.02681-06. Epub 2007 Feb 14. J Virol. 2007. PMID: 17301155 Free PMC article. - An unconventional pathway of mRNA cap formation by vesiculoviruses.
Ogino T, Banerjee AK. Ogino T, et al. Virus Res. 2011 Dec;162(1-2):100-9. doi: 10.1016/j.virusres.2011.09.012. Epub 2011 Sep 16. Virus Res. 2011. PMID: 21945214 Free PMC article. Review. - Flavivirus methyltransferase: a novel antiviral target.
Dong H, Zhang B, Shi PY. Dong H, et al. Antiviral Res. 2008 Oct;80(1):1-10. doi: 10.1016/j.antiviral.2008.05.003. Epub 2008 Jun 5. Antiviral Res. 2008. PMID: 18571739 Free PMC article. Review.
Cited by
- An integrative platform for detection of RNA 2'-O-methylation reveals its broad distribution on mRNA.
Tang Y, Wu Y, Wang S, Lu X, Gu X, Li Y, Yang F, Xu R, Wang T, Jiao Z, Wu Y, Liu L, Chen JQ, Wang Q, Chen Q. Tang Y, et al. Cell Rep Methods. 2024 Mar 25;4(3):100721. doi: 10.1016/j.crmeth.2024.100721. Epub 2024 Mar 6. Cell Rep Methods. 2024. PMID: 38452769 Free PMC article. - Methyltransferase-defective avian metapneumovirus vaccines provide complete protection against challenge with the homologous Colorado strain and the heterologous Minnesota strain.
Sun J, Wei Y, Rauf A, Zhang Y, Ma Y, Zhang X, Shilo K, Yu Q, Saif YM, Lu X, Yu L, Li J. Sun J, et al. J Virol. 2014 Nov;88(21):12348-63. doi: 10.1128/JVI.01095-14. Epub 2014 Aug 13. J Virol. 2014. PMID: 25122790 Free PMC article. - The methyltransferase domain of the Respiratory Syncytial Virus L protein catalyzes cap N7 and 2'-O-methylation.
Sutto-Ortiz P, Tcherniuk S, Ysebaert N, Abeywickrema P, Noël M, Decombe A, Debart F, Vasseur JJ, Canard B, Roymans D, Rigaux P, Eléouët JF, Decroly E. Sutto-Ortiz P, et al. PLoS Pathog. 2021 May 6;17(5):e1009562. doi: 10.1371/journal.ppat.1009562. eCollection 2021 May. PLoS Pathog. 2021. PMID: 33956914 Free PMC article. - Replication-competent vesicular stomatitis virus vaccine vector protects against SARS-CoV-2-mediated pathogenesis.
Case JB, Rothlauf PW, Chen RE, Kafai NM, Fox JM, Shrihari S, McCune BT, Harvey IB, Smith B, Keeler SP, Bloyet LM, Winkler ES, Holtzman MJ, Fremont DH, Whelan SPJ, Diamond MS. Case JB, et al. bioRxiv [Preprint]. 2020 Jul 10:2020.07.09.196386. doi: 10.1101/2020.07.09.196386. bioRxiv. 2020. PMID: 32676597 Free PMC article. Updated. Preprint. - Rational design of human metapneumovirus live attenuated vaccine candidates by inhibiting viral mRNA cap methyltransferase.
Zhang Y, Wei Y, Zhang X, Cai H, Niewiesk S, Li J. Zhang Y, et al. J Virol. 2014 Oct;88(19):11411-29. doi: 10.1128/JVI.00876-14. Epub 2014 Jul 23. J Virol. 2014. PMID: 25056882 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI090060/AI/NIAID NIH HHS/United States
- AI090060/AI/NIAID NIH HHS/United States
- R37 AI059371/AI/NIAID NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- R56 AI090060/AI/NIAID NIH HHS/United States
- AI059371/AI/NIAID NIH HHS/United States
- R01 AI059371/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous