Insulin receptor signaling in normal and insulin-resistant states - PubMed (original) (raw)
Review
Insulin receptor signaling in normal and insulin-resistant states
Jérémie Boucher et al. Cold Spring Harb Perspect Biol. 2014.
Abstract
In the wake of the worldwide increase in type-2 diabetes, a major focus of research is understanding the signaling pathways impacting this disease. Insulin signaling regulates glucose, lipid, and energy homeostasis, predominantly via action on liver, skeletal muscle, and adipose tissue. Precise modulation of this pathway is vital for adaption as the individual moves from the fed to the fasted state. The positive and negative modulators acting on different steps of the signaling pathway, as well as the diversity of protein isoform interaction, ensure a proper and coordinated biological response to insulin in different tissues. Whereas genetic mutations are causes of rare and severe insulin resistance, obesity can lead to insulin resistance through a variety of mechanisms. Understanding these pathways is essential for development of new drugs to treat diabetes, metabolic syndrome, and their complications.
Figures
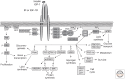
Figure 1.
Insulin- and IGF-1-signaling pathways. Activation of insulin and IGF-1 receptors by their ligands initiates a cascade of phosphorylation events. A conformational change and autophosphorylation of the receptors occur at the time of ligand binding, leading to the recruitment and phosphorylation of receptor substrates such as IRS and Shc proteins. Shc activates the Ras-MAPK pathway, whereas IRS proteins mostly activate the PI3K-Akt pathway by recruiting and activating PI3K, leading to the generation of second messenger PIP3. Membrane-bound PIP3 recruits and activates PDK-1, which phosphorylates and activates Akt and atypical PKCs. Akt mediates most of insulin's metabolic effects, regulating glucose transport, lipid synthesis, gluconeogenesis, and glycogen synthesis. Akt also plays a role in the control of cell cycle and survival. The Shc-Grb2-Sos-Ras-Raf-MAPK pathway controls cellular proliferation and gene transcription.
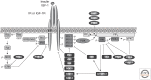
Figure 2.
Negative modulators of insulin and IGF-1 signaling. Intensity and duration of insulin and IGF-1 signaling play an important role in determining the specificity and the nature of the response to these hormones. Signaling is attenuated by action of several phosphatases, which dephosphorylate the receptors, IRS proteins, PKCs, and ERK or PIP3. In addition, stress kinases such as JNK, IKK, and ERK, as well as PKCs or S6K, inhibit insulin/IGF-1 signaling by inducing inhibitory serine/threonine phosphorylation of IR/IGFR and IRS proteins. Trb3 inhibits Akt, and adaptor proteins such as SOCS and Grb bind to the receptors and IRS proteins and inhibit signaling by competition.
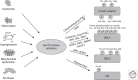
Figure 3.
Activation of Ser/Thr kinases causes inhibitory phosphorylation on insulin-signaling molecules. Lipotoxicity, inflammation, hyperglycemia, and subsequently oxidative stress, as well as mitochondrial dysfunction and ER stress, all converge on activation of Ser/Thr kinases, inducing inhibitory Ser/Thr phosphorylation of IR, IRS proteins, and Akt on multiple residues, causing insulin resistance.
Similar articles
- Role of reduced insulin-stimulated bone blood flow in the pathogenesis of metabolic insulin resistance and diabetic bone fragility.
Hinton PS. Hinton PS. Med Hypotheses. 2016 Aug;93:81-6. doi: 10.1016/j.mehy.2016.05.008. Epub 2016 May 12. Med Hypotheses. 2016. PMID: 27372862 - Intracellular pH Regulation of Skeletal Muscle in the Milieu of Insulin Signaling.
Posa DK, Baba SP. Posa DK, et al. Nutrients. 2020 Sep 23;12(10):2910. doi: 10.3390/nu12102910. Nutrients. 2020. PMID: 32977552 Free PMC article. Review. - Insulin action at a molecular level - 100 years of progress.
White MF, Kahn CR. White MF, et al. Mol Metab. 2021 Oct;52:101304. doi: 10.1016/j.molmet.2021.101304. Epub 2021 Jul 15. Mol Metab. 2021. PMID: 34274528 Free PMC article. Review. - Adipose tissue compensates for defect of phosphatidylinositol 3'-kinase induced in liver and muscle by dietary fish oil in fed rats.
Corporeau C, Foll CL, Taouis M, Gouygou JP, Bergé JP, Delarue J. Corporeau C, et al. Am J Physiol Endocrinol Metab. 2006 Jan;290(1):E78-E86. doi: 10.1152/ajpendo.00200.2005. Am J Physiol Endocrinol Metab. 2006. PMID: 16339925
Cited by
- Therapeutic Effects of Intermittent Fasting Combined with SLBZS and Prebiotics on STZ-HFD-Induced Type 2 Diabetic Mice.
Liu X, Du P, Xu J, Wang W, Zhang C. Liu X, et al. Diabetes Metab Syndr Obes. 2024 Oct 29;17:4013-4030. doi: 10.2147/DMSO.S474196. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39492963 Free PMC article. - Pharmacology of P2X Receptors and Their Possible Therapeutic Potential in Obesity and Diabetes.
Cabral-García GA, Cruz-Muñoz JR, Valdez-Morales EE, Barajas-Espinosa A, Liñán-Rico A, Guerrero-Alba R. Cabral-García GA, et al. Pharmaceuticals (Basel). 2024 Sep 28;17(10):1291. doi: 10.3390/ph17101291. Pharmaceuticals (Basel). 2024. PMID: 39458933 Free PMC article. Review. - Association of Serum Uric Acid with Indices of Insulin Resistance: Proposal of a New Model with Reference to Gender Differences.
Liu R, Li Z, Zhang Y, Du M, Wang X, Zhang S, Li C. Liu R, et al. Diabetes Metab Syndr Obes. 2024 Oct 16;17:3783-3793. doi: 10.2147/DMSO.S481233. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39430137 Free PMC article. - "Therapeutic advancements in nanomedicine: The multifaceted roles of silver nanoparticles".
K Karunakar K, Cheriyan BV, R K, M G, B A. K Karunakar K, et al. Biotechnol Notes. 2024 Jun 1;5:64-79. doi: 10.1016/j.biotno.2024.05.002. eCollection 2024. Biotechnol Notes. 2024. PMID: 39416696 Free PMC article. Review. - The Interlinking Metabolic Association between Type 2 Diabetes Mellitus and Cancer: Molecular Mechanisms and Therapeutic Insights.
Asiri A, Al Qarni A, Bakillah A. Asiri A, et al. Diagnostics (Basel). 2024 Sep 25;14(19):2132. doi: 10.3390/diagnostics14192132. Diagnostics (Basel). 2024. PMID: 39410536 Free PMC article. Review.
References
- Aguirre V, Uchida T, Yenush L, Davis R, White MF 2000. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054 - PubMed
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF 2002. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537 - PubMed
- Akira S, Takeda K 2004. Toll-like receptor signalling. Nat Rev Immunol 4: 499–511 - PubMed
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P 1997. Characterization of a 3-phosphoinositide-dependent protein kinase, which phosphorylates and activates protein kinase Bα. Curr Biol 7: 261–269 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R01 DK033201/DK/NIDDK NIH HHS/United States
- R01 DK055545/DK/NIDDK NIH HHS/United States
- R37 DK031036/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources