Functional analysis of the interdependence between DNA uptake sequence and its cognate ComP receptor during natural transformation in Neisseria species - PubMed (original) (raw)
Functional analysis of the interdependence between DNA uptake sequence and its cognate ComP receptor during natural transformation in Neisseria species
Jamie-Lee Berry et al. PLoS Genet. 2013.
Abstract
Natural transformation is the widespread biological process by which "competent" bacteria take up free DNA, incorporate it into their genomes, and become genetically altered or "transformed". To curb often deleterious transformation by foreign DNA, several competent species preferentially take up their own DNA that contains specific DUS (DNA uptake sequence) watermarks. Our recent finding that ComP is the long sought DUS receptor in Neisseria species paves the way for the functional analysis of the DUS-ComP interdependence which is reported here. By abolishing/modulating ComP levels in Neisseria meningitidis, we show that the enhancement of transformation seen in the presence of DUS is entirely dependent on ComP, which also controls transformation in the absence of DUS. While peripheral bases in the DUS were found to be less important, inner bases are essential since single base mutations led to dramatically impaired interaction with ComP and transformation. Strikingly, naturally occurring DUS variants in the genomes of human Neisseria commensals differing from DUS by only one or two bases were found to be similarly impaired for transformation of N. meningitidis. By showing that ComPsub from the N. subflava commensal specifically binds its cognate DUS variant and mediates DUS-enhanced transformation when expressed in a comP mutant of N. meningitidis, we confirm that a similar mechanism is used by all Neisseria species to promote transformation by their own, or closely related DNA. Together, these findings shed new light on the molecular events involved in the earliest step in natural transformation, and reveal an elegant mechanism for modulating horizontal gene transfer between competent species sharing the same niche.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
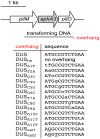
Figure 1. Schematic cartoon, drawn to scale, of transforming DNAs used in this study.
PCR fragments (1.9 kbp) corresponding to a ΔpilN mutation were used for transformations. In ΔpilN, an aphA3 cassette encoding resistance to kanamycin replaces the pilN gene and is flanked by 542 and 597 bp of pilM and pilO, respectively . This fragment contains no canonical DUS (ATGCCGTCTGAA), hence DUS, or DUS mutants/variants whose base composition can be precisely controlled, can be added in 3′ by using reverse primers containing the listed overhangs.
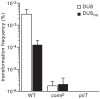
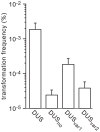
Figure 2. DUS enhancement of transformation in N. meningitidis 8013 is significant and ComP-dependent.
Competence for DNA transformation in strain 8013 and its isogenic comP and pilT mutants was quantified by transforming equivalent numbers of recipient cells with 100 ng of ΔpilN PCR fragments obtained using primers containing the canonical DUS (white bars), or no DUS (black bars). Recipient cells and KmR transformants were counted by plating. Results are expressed as percentages of recipient cells transformed, and are the mean ± standard deviation of six independent experiments.
Figure 3. ComP controls transformation both in the presence and in the absence of DUS.
(A) Immunoblot analysis of ComP production (upper panel) in strain comP/comPind in the presence of increasing concentrations of IPTG. Strain comP/comPind is a comP mutant complemented by an ectopic WT copy of comP under the control of an IPTG-inducible promoter. The WT strain was included as a control. Equal amounts of whole-cell protein extracts were loaded in each lane, which was confirmed by detecting PilW (lower panel). (B) Quantification of competence for DNA transformation in strain comP/comPind in the presence of increasing concentrations of IPTG using ΔpilN PCR fragments obtained with primers containing the canonical DUS (white bars), or no DUS (black bars). The WT strain was included as a control. Results are expressed as percentages of recipient cells transformed, and are the mean ± standard deviation of four to six independent experiments.
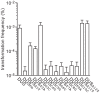
Figure 4. Seven inner bases of DUS are critical for enhancement of transformation.
Competence for DNA transformation in N. meningitidis 8013 was quantified after transformation with 100 ng of ΔpilN PCR fragments containing a series of DUS mutants in which each base of the DUS was mutated. The specific transversion mutations are indicated in each case. As a control, the WT strain transformed with DUS and no DUS was included. Results are expressed as percentages of recipient cells transformed, and are the mean ± standard deviation of six independent experiments.
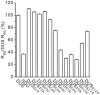
Figure 5. Most inner bases of DUS critical for enhancement of transformation are also critical for recognition by ComP.
Similar amounts (as assessed by measuring RU) of biotinylated ds primers corresponding to DUS, SDU (a primer in which every second base of DUS is changed) and the various DUS mutants were immobilized on sensor chips. For each DNA, Req values were determined with 30 µM of pure ComP and normalized to bound RU. Results are expressed in % as ratios to DUS Req, and are the mean ± standard deviation of 12 independent experiments.
Figure 6. Naturally occuring DUS variants in human Neisseria commensals are impaired for enhancement of transformation in N. meningitidis.
The ability of DUSvar1 and DUSvar2 to transform N. meningitidis 8013 was quantified using 100 ng of ΔpilN PCR fragments containing these DUS variants and compared to that of DUS and DUSno. Results are expressed as percentages of recipient cells transformed, and are the mean ± standard deviation of six to seven independent experiments.
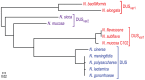
Figure 7. Human Neisseria species harbouring different DUS variants have different cognate ComPs.
A maximum likelihood phylogenetic tree based on the sequences of mature ComP found in human Neisseria species shows that species harbouring the same DUS (highlighted in the same colour) have highly conserved ComPs.
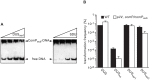
Figure 8. ComP homologs in other human Neisseria species are DNA receptors playing a similar role in transformation to meningococcal ComP.
(A) Effect on the ComPsub-DNA complex of the addition of increasing amounts of unlabelled competitor as assessed by acrylamide EMSA. ComPsub and biotin-labelled PCR fragment containing one copy of DUSvar1 were incubated with two-fold increasing concentrations of unlabelled ds DUSvar1 or SDU primers. DNA was then resolved by electrophoresis on an acrylamide native gel, transferred to a positive nylon membrane, UV-crosslinked and detected using a streptavidin-HRP conjugate. (B) Quantification of competence for DNA transformation in the strain 8013 (black bars) and an engineered derivative expressing ComPsub (white bars). Strains were transformed in the presence of 500 µM IPTG using 10 ng of plasmids in which ΔpilN mutant PCR fragments containing DUS, DUSno, DUSvar1 and DUSvar2 have been cloned. Results are expressed as percentages of recipient cells transformed, and are the mean ± standard deviation of five independent experiments.
Similar articles
- New functional identity for the DNA uptake sequence in transformation and its presence in transcriptional terminators.
Ambur OH, Frye SA, Tønjum T. Ambur OH, et al. J Bacteriol. 2007 Mar;189(5):2077-85. doi: 10.1128/JB.01408-06. Epub 2006 Dec 28. J Bacteriol. 2007. PMID: 17194793 Free PMC article. - DNA uptake sequence-mediated enhancement of transformation in Neisseria gonorrhoeae is strain dependent.
Duffin PM, Seifert HS. Duffin PM, et al. J Bacteriol. 2010 Sep;192(17):4436-44. doi: 10.1128/JB.00442-10. Epub 2010 Jul 2. J Bacteriol. 2010. PMID: 20601472 Free PMC article. - Restriction and sequence alterations affect DNA uptake sequence-dependent transformation in Neisseria meningitidis.
Ambur OH, Frye SA, Nilsen M, Hovland E, Tønjum T. Ambur OH, et al. PLoS One. 2012;7(7):e39742. doi: 10.1371/journal.pone.0039742. Epub 2012 Jul 2. PLoS One. 2012. PMID: 22768309 Free PMC article. - The genetics of Neisseria species.
Rotman E, Seifert HS. Rotman E, et al. Annu Rev Genet. 2014;48:405-31. doi: 10.1146/annurev-genet-120213-092007. Epub 2014 Sep 10. Annu Rev Genet. 2014. PMID: 25251852 Review. - Mechanisms of DNA Uptake by Naturally Competent Bacteria.
Dubnau D, Blokesch M. Dubnau D, et al. Annu Rev Genet. 2019 Dec 3;53:217-237. doi: 10.1146/annurev-genet-112618-043641. Epub 2019 Aug 21. Annu Rev Genet. 2019. PMID: 31433955 Review.
Cited by
- Natural competence and the evolution of DNA uptake specificity.
Mell JC, Redfield RJ. Mell JC, et al. J Bacteriol. 2014 Apr;196(8):1471-83. doi: 10.1128/JB.01293-13. Epub 2014 Jan 31. J Bacteriol. 2014. PMID: 24488316 Free PMC article. Review. - Virulence genes and previously unexplored gene clusters in four commensal Neisseria spp. isolated from the human throat expand the neisserial gene repertoire.
Calder A, Menkiti CJ, Çağdaş A, Lisboa Santos J, Streich R, Wong A, Avini AH, Bojang E, Yogamanoharan K, Sivanesan N, Ali B, Ashrafi M, Issa A, Kaur T, Latif A, Mohamed HAS, Maqsood A, Tamang L, Swager E, Stringer AJ, Snyder LAS. Calder A, et al. Microb Genom. 2020 Sep;6(9):mgen000423. doi: 10.1099/mgen.0.000423. Epub 2020 Aug 26. Microb Genom. 2020. PMID: 32845827 Free PMC article. - Microevolution and Its Impact on Hypervirulence, Antimicrobial Resistance, and Vaccine Escape in Neisseria meningitidis.
Mikucki A, Kahler CM. Mikucki A, et al. Microorganisms. 2023 Dec 18;11(12):3005. doi: 10.3390/microorganisms11123005. Microorganisms. 2023. PMID: 38138149 Free PMC article. Review. - Editorial.
Filloux A. Filloux A. FEMS Microbiol Rev. 2015 Jan;39(1):1. doi: 10.1093/femsre/fuu005. FEMS Microbiol Rev. 2015. PMID: 25793962 No abstract available. - The molecular basis of FimT-mediated DNA uptake during bacterial natural transformation.
Braus SAG, Short FL, Holz S, Stedman MJM, Gossert AD, Hospenthal MK. Braus SAG, et al. Nat Commun. 2022 Mar 4;13(1):1065. doi: 10.1038/s41467-022-28690-1. Nat Commun. 2022. PMID: 35246533 Free PMC article.
References
- Johnsborg O, Eldholm V, Håvarstein LS (2007) Natural genetic transformation: prevalence, mechanisms and function. Res Microbiol 158: 767–778. - PubMed
- Virji M (2009) Pathogenic Neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol 7: 274–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 100298/WT_/Wellcome Trust/United Kingdom
- MR/J006874/1/MRC_/Medical Research Council/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous