Meiosis and haploid gametes in the pathogen Trypanosoma brucei - PubMed (original) (raw)
Meiosis and haploid gametes in the pathogen Trypanosoma brucei
Lori Peacock et al. Curr Biol. 2014.
Abstract
In eukaryote pathogens, sex is an important driving force in spreading genes for drug resistance, pathogenicity, and virulence. For the parasitic trypanosomes that cause African sleeping sickness, mating occurs during transmission by the tsetse vector and involves meiosis, but haploid gametes have not yet been identified. Here, we show that meiosis is a normal part of development in the insect salivary glands for all subspecies of Trypanosoma brucei, including the human pathogens. By observing insect-derived trypanosomes during the window of peak expression of meiosis-specific genes, we identified promastigote-like (PL) cells that interacted with each other via their flagella and underwent fusion, as visualized by the mixing of cytoplasmic red and green fluorescent proteins. PL cells had a short, wide body, a very long anterior flagellum, and either one or two kinetoplasts, but only the anterior kinetoplast was associated with the flagellum. Measurement of nuclear DNA contents showed that PL cells were haploid relative to diploid metacyclics. Trypanosomes are among the earliest diverging eukaryotes, and our results support the hypothesis that meiosis and sexual reproduction are ubiquitous in eukaryotes and likely to have been early innovations.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
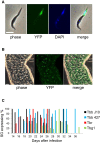
Figure 1
Expression of Meiosis-Specific YFP Fusion Proteins in Trypanosomes from Salivary Glands of Tsetse Flies (A) Expression of YFP::DMC1 in T. b. gambiense DAL972. Scale bar, 5 μm. (B) Part of a tsetse salivary gland infected with T. b. brucei 427 var 3; live image. Many attached trypanosomes are expressing YFP::HOP1. Scale bar, 20 μm. (C) Aggregated results of expression of meiosis-specific genes over time. Flies were dissected from 14 to 38 days after infection and scored positive if the salivary glands contained a minimum of one trypanosome expressing a meiosis-specific gene (MND1, DMC1, or HOP1). The color denotes the trypanosome isolate.
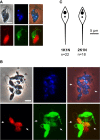
Figure 2
Interactions and Cytoplasmic Exchange between Salivary Gland-Derived Trypanosomes Ex Vivo Mix of trypanosome clones F1G1 (green fluorescence) and F1R1 (red fluorescence). (A) Pair of interacting trypanosomes; both are 2K1N promastigote-like (PL) cells; note the intertwined flagella and close proximity of cell bodies. Scale bar, 5 μm. (B) Cluster of mating trypanosomes; two trypanosomes (arrows) have exchanged cytoplasm, demonstrated by both green and red fluorescence. Scale bar, 10 μm. (C) Diagram compiled from measurements of 1K1N and 2K1N PL cells of 1738 GFP from salivary glands dissected 20 days after infection. See also Movies S2, S3, and S4.
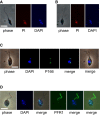
Figure 3
Morphology of 2K1N Promastigote-like Trypanosomes (A and B) Differential staining of DNA with DAPI and PI. DAPI preferentially binds to AT-rich DNA compared to PI, allowing the AT-rich kinetoplast DNA to be distinguished from nuclear DNA. Two different 2K1N PL cells are shown. (C) Localization of protein P166 from tripartite attachment complex in 2K1N PL cell of T. b. brucei J10 carrying fusion construct YFP::P166. (D) Visualization of flagellum in 2K1N PL cell of T. b. brucei J10 carrying fusion construct YFP::PFR1. The trypanosome has a single flagellum associated with the anterior kinetoplast. Scale bars, 10 μm. See also Figure S1.
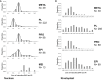
Figure 4
Nuclear and Kinetoplast DNA Contents of Salivary Gland-Derived Trypanosomes Trypanosomes (1738 GFP) from flies dissected 17–20 days after infection were fixed and stained with DAPI and PI before imaging under uniform conditions. Total pixel intensities were measured for both DAPI and PI-stained nuclei and kinetoplasts using ImageJ (
) and normalized relative to G1-arrested metacyclics (META, nuclear DNA content = 1.0; kinetoplast DNA content = 1.0). Other cells were categorized by morphology into promastigote-like cells (PL; see Figure 2C), procyclics (PRO; long trypomastigote with round or oval nucleus), epimastigotes (EPI; nucleus posterior to kinetoplast), cells in meiosis I (MEI; epimastigote with posterior nucleus, two anterior kinetoplasts and associated flagella). N denotes number of organelles measured in each cell category. (A) Nuclear DNA contents. Each histogram shows the frequency distribution of total normalized pixel intensities per nucleus; values on the x axis are the median for each bin (bin size = 0.25). The superimposed dotted line is a smooth curve. (B) Kinetoplast DNA contents. Each histogram shows the frequency distribution of total normalized pixel intensities per kinetoplast; values on the x axis are the median for each bin (bin size = 0.25).
Similar articles
- Mating compatibility in the parasitic protist Trypanosoma brucei.
Peacock L, Ferris V, Bailey M, Gibson W. Peacock L, et al. Parasit Vectors. 2014 Feb 21;7:78. doi: 10.1186/1756-3305-7-78. Parasit Vectors. 2014. PMID: 24559099 Free PMC article. - Sequential production of gametes during meiosis in trypanosomes.
Peacock L, Kay C, Farren C, Bailey M, Carrington M, Gibson W. Peacock L, et al. Commun Biol. 2021 May 11;4(1):555. doi: 10.1038/s42003-021-02058-5. Commun Biol. 2021. PMID: 33976359 Free PMC article. - Evidence for haploidy in metacyclic forms of Trypanosoma brucei.
Zampetti-Bosseler F, Schweizer J, Pays E, Jenni L, Steinert M. Zampetti-Bosseler F, et al. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6063-4. doi: 10.1073/pnas.83.16.6063. Proc Natl Acad Sci U S A. 1986. PMID: 3461475 Free PMC article. - Liaisons dangereuses: sexual recombination among pathogenic trypanosomes.
Gibson W. Gibson W. Res Microbiol. 2015 Jul-Aug;166(6):459-66. doi: 10.1016/j.resmic.2015.05.005. Epub 2015 May 28. Res Microbiol. 2015. PMID: 26027775 Review. - The flagellum of Trypanosoma brucei: new tricks from an old dog.
Ralston KS, Hill KL. Ralston KS, et al. Int J Parasitol. 2008 Jul;38(8-9):869-84. doi: 10.1016/j.ijpara.2008.03.003. Epub 2008 Mar 28. Int J Parasitol. 2008. PMID: 18472102 Free PMC article. Review.
Cited by
- What do isogamous organisms teach us about sex and the two sexes?
Lehtonen J, Kokko H, Parker GA. Lehtonen J, et al. Philos Trans R Soc Lond B Biol Sci. 2016 Oct 19;371(1706):20150532. doi: 10.1098/rstb.2015.0532. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27619696 Free PMC article. Review. - Fluorescent proteins reveal what trypanosomes get up to inside the tsetse fly.
Gibson W, Peacock L. Gibson W, et al. Parasit Vectors. 2019 Jan 4;12(1):6. doi: 10.1186/s13071-018-3204-y. Parasit Vectors. 2019. PMID: 30609932 Free PMC article. Review. - Sexual reproduction in a natural Trypanosoma cruzi population.
Berry ASF, Salazar-Sánchez R, Castillo-Neyra R, Borrini-Mayorí K, Chipana-Ramos C, Vargas-Maquera M, Ancca-Juarez J, Náquira-Velarde C, Levy MZ, Brisson D; Chagas Disease Working Group in Arequipa. Berry ASF, et al. PLoS Negl Trop Dis. 2019 May 20;13(5):e0007392. doi: 10.1371/journal.pntd.0007392. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31107905 Free PMC article. - HOP1 and HAP2 are conserved components of the meiosis-related machinery required for successful mating in Leishmania.
Catta-Preta CMC, Ferreira TR, Ghosh K, Paun A, Sacks D. Catta-Preta CMC, et al. Nat Commun. 2023 Nov 7;14(1):7159. doi: 10.1038/s41467-023-42789-z. Nat Commun. 2023. PMID: 37935664 Free PMC article. - Life-cycle modification in open oceans accounts for genome variability in a cosmopolitan phytoplankton.
von Dassow P, John U, Ogata H, Probert I, Bendif el M, Kegel JU, Audic S, Wincker P, Da Silva C, Claverie JM, Doney S, Glover DM, Flores DM, Herrera Y, Lescot M, Garet-Delmas MJ, de Vargas C. von Dassow P, et al. ISME J. 2015 Jun;9(6):1365-77. doi: 10.1038/ismej.2014.221. Epub 2014 Dec 2. ISME J. 2015. PMID: 25461969 Free PMC article.
References
- Jenni L., Marti S., Schweizer J., Betschart B., Le Page R.W.F., Wells J.M., Tait A., Paindavoine P., Pays E., Steinert M. Hybrid formation between African trypanosomes during cyclical transmission. Nature. 1986;322:173–175. - PubMed
- Ramesh M.A., Malik S.B., Logsdon J.M., Jr. A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr. Biol. 2005;15:185–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources