Enhanced ubiquitin-dependent degradation by Nedd4 protects against α-synuclein accumulation and toxicity in animal models of Parkinson's disease - PubMed (original) (raw)
Enhanced ubiquitin-dependent degradation by Nedd4 protects against α-synuclein accumulation and toxicity in animal models of Parkinson's disease
Sian E Davies et al. Neurobiol Dis. 2014 Apr.
Abstract
Parkinson's disease is a neurodegenerative disorder, characterized by accumulation and misfolding of α-synuclein. Although the level of α-synuclein in neurons is fundamentally linked to the onset of neurodegeneration, multiple pathways have been implicated in its degradation, and it remains unclear which are the critical ubiquitination enzymes that protect against α-synuclein accumulation in vivo. The ubiquitin ligase Nedd4 targets α-synuclein to the endosomal-lysosomal pathway in cultured cells. Here we asked whether Nedd4-mediated degradation protects against α-synuclein-induced toxicity in the Drosophila and rodent models of Parkinson's disease. We show that overexpression of Nedd4 can rescue the degenerative phenotype from ectopic expression of α-synuclein in the Drosophila eye. Overexpressed Nedd4 in the Drosophila brain prevented the α-synuclein-induced locomotor defect whereas reduction in endogenous Nedd4 by RNAi led to worsening motor function and increased loss of dopaminergic neurons. Accordingly, AAV-mediated expression of wild-type but not the catalytically inactive Nedd4 decreased the α-synuclein-induced dopaminergic cell loss in the rat substantia nigra and reduced α-synuclein accumulation. Collectively, our data in two evolutionarily distant model organisms strongly suggest that Nedd4 is a modifier of α-synuclein pathobiology and thus a potential target for neuroprotective therapies.
Keywords: Endosomal-trafficking; Lysosome; Protein-degradation; Ubiquitination.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Fig. 1
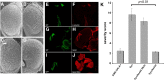
Eye-specific expression of α-synuclein by GMR-GAL4 causes a rough eye phenotype that is prevented by Nedd4 co-expression. Unlike control flies expressing GMR-GAL4/+ (A), overexpression of α-synuclein caused a rough eye phenotype (B) that is detectable only by SEM. (C) The phenotype was not worsened in doubly transgenic flies co-expressing Nedd4 RNAi and α-synuclein (labeled Syn/Nedd4 RNAi). (D) Overproduction of Nedd4 prevented the α-synuclein-induced degenerative eye phenotype (labeled Syn/Nedd4). Scale bar, 100 μm. ‘Flip-out’ clones confirmed that α-synuclein (E, F) Nedd4 (G, H) and Nedd4 RNAi (I, J) were readily expressed in GFP positive clones in larval eye discs upon heat shock. (K) Quantitative analysis of the severity of the eye phenotypes; n = 20 SEM images per group, p < 0.01 using ANOVA.
Fig. 2
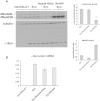
Increased neuronal Nedd4 reduces α-synuclein levels in the Drosophila brain. (A) Representative immunoblot with three independent samples from each transgenic line: _elav-_GAL4/+, α-synuclein flies (Syn) and doubly transgenic α-synuclein flies with either Nedd4 RNAi (labeled Syn/Nedd4 RNAi) or Nedd4 overexpression (labeled Syn/Nedd4). Quantitative band densitometry showed that, relative to tubulin loading control, neuronal α-synuclein was significantly reduced by 2-fold in flies co-expressing Nedd4 (upper histogram). Neuronal expression of Nedd4 was reduced by 2-fold in RNAi expressing flies and increased by 5–6 fold in flies expressing the short isoform of Nedd4 (lower histogram). Values represent mean ± SEM from three independent experiments. (B) Quantitative PCR did not show a significant difference in α-synuclein mRNA levels between the three transgenic lines and no signal was detected in the control genotype as expected.
Fig. 3
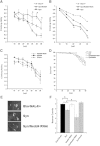
Nedd4 levels modify the α-synuclein-induced motor phenotype and dopaminergic cell number in Drosophila. (A) Accelerated loss of climbing ability was seen in transgenic flies expressing α-synuclein throughout the nervous system (_elav-_GAL4 driver). The climbing ability of doubly transgenic lines expressing Nedd4 and α-synuclein was significantly improved (shown by asterisks) when compared to α-synuclein expressing flies and was similar to the control genotype, _elav-_GAL4/+. (B) Unlike the result in the eye, reduction of endogenous Nedd4 specifically in the nervous system caused a worsening in the climbing scores, which were significantly different between doubly transgenic Nedd4 RNAi/α-synuclein compared to either α-synuclein or the control genotype, elav-GAL4/+ (error bars are hidden within the symbols). (C) No significant difference was detected when Nedd4 per se was overexpressed or knocked-down. *p < 0.05 using ANOVA with subsequent Tukey–Kramer test. Values represent mean ± SEM. (D) Kaplan–Meier survival curves were similar at different days after eclosion (DAE). (E) Dopaminergic neurons in the dorsomedial cluster were visualized with anti-TH antibodies and counted. (F) A significant loss of dopaminergic neurons was seen in Nedd4 RNAi/α-synuclein doubly transgenic flies when compared to α-synuclein or elav-GAL4/+ phenotypes. Co-expression of Nedd4 was protective against α-synuclein-induced cell loss (n = 10 brains/group). *p < 0.05 using ANOVA with subsequent Tukey–Kramer test. Values represent mean ± SEM.
Fig. 4
Overexpression of wild-type but not catalytically inactive Nedd4 prevents α-synuclein-induced loss of dopaminergic neurons. Virally-mediated overexpression of human A53T α-synuclein at 19 weeks post-AAV injection was confirmed using the LB509 antibody (panel A), and Nedd4 overexpression was verified using anti-T7 (panel B). TH was used to label dopaminergic neurons (panel C). Co-expression of both Nedd4 and human α-synuclein within TH-immunoreactive SNc neurons is shown in panel D, and represents the boxed area illustrated in panel A. Examples of TH-immunoreactive neurons expressing both transgenes are marked with asterisks. Expression was also confirmed by immunoblotting of SN lysates at 19 weeks with antibodies against α-synuclein (Syn1) and T7 for Nedd4 (panel E). Representative images and high magnification insets are shown in panels F–H for TH/NeuN doubly labeled neurons in the SNc for each group. (I) When compared to AAV-GFP controls (n = 5), there was a significant reduction in the number of dopaminergic neurons in rats expressing Nedd4C-S and A53T α-synuclein (n = 8) whereas co-expression of Nedd4WT (n = 7) afforded protection. *p < 0.05, p < 0.001 (one-way ANOVA, Tukey's multiple comparison post-hoc test). Scale bar, panels A, B = 200 μm, panel D = 50 μm, panels F–G = 100 μm, insets = 50 μm.
Fig. 5
Wild-type but not catalytically inactive Nedd4 prevents α-synuclein accumulation in the rat SN. The level of cytosolic and aggregated α-synuclein was quantified following serial fractionation and immunoblotting of SN lysates at 19 weeks post-AAV injection of either A53T α-synuclein/Nedd4WT or A53T α-synuclein/Nedd4C-S. (A) Representative immunoblot showing cytosolic and aggregated α-synuclein at 19 weeks post-AAV injection for three animals per group. Actin is shown as loading control. Quantitative band densitometry (n = 5–6 animals per group) showed that the cytosolic α-synuclein content was reduced by 33% in rats co-expressing Nedd4WT (panel A, upper histogram) whereas the RIPA-insoluble/SDS soluble (gel excluded and oligomeric) α-synuclein, was significantly increased by 3.6-fold in rats co-expressing Nedd4C-S (panel A, lower histogram). B. Recombinant wild-type and A53T mutant α-synuclein was incubated with purified Nedd4, UbcH5 as the E2 with or without ubiquitin, and ubiquitination was assayed using anti-α-synuclein antibodies. Polyubiquitination (syn + pUb) of both recombinant WT and A53T mutant α-synuclein by Nedd4 was detected. High molecular weight species of aggregated A53T α-synuclein, present in the reaction before ubiquitin was added, were also ubiquitinated by Nedd4 as evidenced by the increase in their molecular weight upon addition of recombinant ubiquitin. Monomeric α-synuclein was added in excess and thus not clearly depleted in this reaction.
Similar articles
- Lys-63-linked ubiquitination by E3 ubiquitin ligase Nedd4-1 facilitates endosomal sequestration of internalized α-synuclein.
Sugeno N, Hasegawa T, Tanaka N, Fukuda M, Wakabayashi K, Oshima R, Konno M, Miura E, Kikuchi A, Baba T, Anan T, Nakao M, Geisler S, Aoki M, Takeda A. Sugeno N, et al. J Biol Chem. 2014 Jun 27;289(26):18137-51. doi: 10.1074/jbc.M113.529461. Epub 2014 May 15. J Biol Chem. 2014. PMID: 24831002 Free PMC article. - Ubiquitin ligase Nedd4 promotes alpha-synuclein degradation by the endosomal-lysosomal pathway.
Tofaris GK, Kim HT, Hourez R, Jung JW, Kim KP, Goldberg AL. Tofaris GK, et al. Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):17004-9. doi: 10.1073/pnas.1109356108. Epub 2011 Sep 27. Proc Natl Acad Sci U S A. 2011. PMID: 21953697 Free PMC article. - Septin 4, the drosophila ortholog of human CDCrel-1, accumulates in parkin mutant brains and is functionally related to the Nedd4 E3 ubiquitin ligase.
Muñoz-Soriano V, Nieto-Arellano R, Paricio N. Muñoz-Soriano V, et al. J Mol Neurosci. 2012 Sep;48(1):136-43. doi: 10.1007/s12031-012-9788-3. Epub 2012 May 6. J Mol Neurosci. 2012. PMID: 22562816 - The Role of NEDD4 E3 Ubiquitin-Protein Ligases in Parkinson's Disease.
Conway JA, Kinsman G, Kramer ER. Conway JA, et al. Genes (Basel). 2022 Mar 14;13(3):513. doi: 10.3390/genes13030513. Genes (Basel). 2022. PMID: 35328067 Free PMC article. Review. - The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration.
Mor DE, Daniels MJ, Ischiropoulos H. Mor DE, et al. Mov Disord. 2019 Feb;34(2):167-179. doi: 10.1002/mds.27607. Epub 2019 Jan 11. Mov Disord. 2019. PMID: 30633814 Free PMC article. Review.
Cited by
- Ubiquitin and Ubiquitin-like Proteins in Cancer, Neurodegenerative Disorders, and Heart Diseases.
Hwang JT, Lee A, Kho C. Hwang JT, et al. Int J Mol Sci. 2022 May 2;23(9):5053. doi: 10.3390/ijms23095053. Int J Mol Sci. 2022. PMID: 35563444 Free PMC article. Review. - Lys-63-linked ubiquitination by E3 ubiquitin ligase Nedd4-1 facilitates endosomal sequestration of internalized α-synuclein.
Sugeno N, Hasegawa T, Tanaka N, Fukuda M, Wakabayashi K, Oshima R, Konno M, Miura E, Kikuchi A, Baba T, Anan T, Nakao M, Geisler S, Aoki M, Takeda A. Sugeno N, et al. J Biol Chem. 2014 Jun 27;289(26):18137-51. doi: 10.1074/jbc.M113.529461. Epub 2014 May 15. J Biol Chem. 2014. PMID: 24831002 Free PMC article. - Generation of SNCA Cell Models Using Zinc Finger Nuclease (ZFN) Technology for Efficient High-Throughput Drug Screening.
Dansithong W, Paul S, Scoles DR, Pulst SM, Huynh DP. Dansithong W, et al. PLoS One. 2015 Aug 28;10(8):e0136930. doi: 10.1371/journal.pone.0136930. eCollection 2015. PLoS One. 2015. PMID: 26317803 Free PMC article. - Oligomerization of Selective Autophagy Receptors for the Targeting and Degradation of Protein Aggregates.
Chen W, Shen T, Wang L, Lu K. Chen W, et al. Cells. 2021 Aug 5;10(8):1989. doi: 10.3390/cells10081989. Cells. 2021. PMID: 34440758 Free PMC article. Review. - Neuronal Vulnerability to Degeneration in Parkinson's Disease and Therapeutic Approaches.
Sharma T, Kumar R, Mukherjee S. Sharma T, et al. CNS Neurol Disord Drug Targets. 2024;23(6):715-730. doi: 10.2174/1871527322666230426155432. CNS Neurol Disord Drug Targets. 2024. PMID: 37185323 Review.
References
- Auluck P.K., Edwin Chan H.Y., Trojanowski J.Q., Lee V.M.-Y., Bonini N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_U137788471/MRC_/Medical Research Council/United Kingdom
- K-0819/PUK_/Parkinson's UK/United Kingdom
- MC_UU_12021/3/MRC_/Medical Research Council/United Kingdom
- J-0901/PUK_/Parkinson's UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases