HLA-B*57 elite suppressor and chronic progressor HIV-1 isolates replicate vigorously and cause CD4+ T cell depletion in humanized BLT mice - PubMed (original) (raw)
HLA-B*57 elite suppressor and chronic progressor HIV-1 isolates replicate vigorously and cause CD4+ T cell depletion in humanized BLT mice
Maria Salgado et al. J Virol. 2014 Mar.
Abstract
Elite controllers or suppressors (ES) are HIV-1-infected patients who maintain undetectable viral loads without antiretroviral therapy. The mechanism of control remains unclear, but the HLA-B*57 allele is overrepresented in cohorts of these patients. However, many HLA-B*57 patients develop progressive disease, and some studies have suggested that infection with defective viruses may be the cause of the lack of high levels of virus replication and disease progression in ES. We therefore performed a comprehensive comparative in vivo and in vitro characterization of viruses isolated from well-defined ES. For this purpose, we first performed full-genome sequence analysis and in vitro fitness assays on replication-competent isolates from HLA-B*57 ES and HLA-B*57 chronic progressors (CPs). Under our experimental conditions, we found that isolates from ES and CPs can replicate in vitro. However, since inherently these assays involve the use of unnaturally in vitro-activated cells, we also investigated the replication competence and pathogenic potential of these HIV isolates in vivo using humanized BLT mice. The results from these analyses demonstrate that virus isolates from ES are fully replication competent in vivo and can induce peripheral and systemic CD4 T cell depletion. These results provide the first direct in vivo evidence that viral fitness does not likely determine clinical outcome in HLA-B*57 patients and that elite suppressors can control replication-competent, fully pathogenic viruses. A better understanding of the immunological bases of viral suppression in ES will serve to inform novel approaches to preventive and therapeutic HIV vaccine design.
Importance: Elite suppressors are HIV-1-infected patients who have undetectable levels of viremia despite not being on antiviral drugs. One of the most fundamental questions about this phenomenon involves the mechanism of control. To address this question, we isolated virus from elite suppressors and from HIV-1-infected patients who have the usual progressive disease course. We compared how well the isolates from the two groups of patients replicated in culture and in humanized mice. Our results suggest that elite suppressors are capable of controlling HIV-1 due to the possession of unique host factors rather than infection with defective virus.
Figures
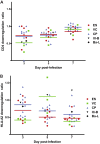
FIG 1
(A) Growth kinetics of 18 HIV-1 isolates cultured from CD4+ T cells from HLA-B*57+ patients. The patients were ES (red), VCs (green), or CPs (blue). Two laboratory strains (black) were included for comparison. The isolates were all cultured in activated CD4+ T cells from the same HIV-1 negative donor. (B) Median growth curve for each group of patients. (C and D) Sequence variation within the three HLA-B*57-03-restricted Gag (C) and Nef (D) epitopes for each isolate. The red and blue boxes denote two distinct but overlapping Nef epitopes, HW9 (116 to 124) and YT9 (120 to 128), respectively. Comparisons of the degree of sequence variation were made using the Mann-Whitney test.
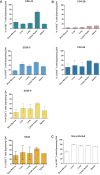
FIG 2
Variations within HLA-B*57-restricted epitopes. Sequence analysis of epitopes in reverse transcriptase (A), integrase (B), Vpr (C), Vif (D), Rev (E), and Env (F) is shown.
FIG 3
Downregulation of CD4 and HLA-A2 in primary CD4+ T cells infected with HIV-1 isolates. The isolates were laboratory isolates (black) or HIV-1 isolates cultured from ES (red), VCs (green), or CPs (blue). Each symbol represents an individual isolate. The HLA-A2 downregulation ratio (A) is the MFI of HLA-A2 on cells that were positive for intracellular Gag divided by the MFI of HLA-A2 on CD4+ T cells that were Gag negative. The CD4 downregulation ratio (B) represents the fraction of all CD4+ T cells that were Gag positive and CD4 low. The horizontal lines represent the median level of downregulation for each group of patients. The Mann-Whitney test was used to compare the degrees of CD4 and HLA-A2 downregulation in the different patient populations.
FIG 4
In vivo replication and pathogenesis of HIV isolates from ES and CPs. BLT humanized mice were exposed to HIV-1 isolates that were cultured from either ES or CPs. Each isolate was used to infect two mice. The mice were bled periodically to obtain plasma for viral load analysis via real-time RT-PCR and blood mononuclear cells for flow cytometric analysis. For each isolate, the left panel shows viral load analysis (the dotted line represents the limit of detection for the viral load assay), and the right panel shows the percentage of peripheral blood CD4+ T cells. Different symbols represent different mice. One mouse infected with isolate CP4-2B and one mouse infected with isolate ES40 died shortly after day 28 and day 42 of infection, respectively.
FIG 5
HIV isolates derived from elite suppressors are pathogenic in vivo and result in systemic CD4+ T cell depletion. Mononuclear cells were isolated from various tissues at necropsy. The percentage of T cells that express CD4 was calculated for each tissue. The bars represent the average for two animals. Results are from BLT mice infected with HLA*B57 elite suppressor-derived isolates (A), HLA*B57 chronic progressor-derived isolates (B), or noninfected BLT mice (C).
FIG 6
Establishment of a latent viral reservoir by an ES-derived HIV isolate. (A) Experimental overview for the ex vivo determination of latency. (B) Longitudinal analysis of plasma viral loads (orange circles) and the levels of CD4+ T cells in the blood (blue circles) of a BLT mouse exposed to the ES-derived isolate ES38-9. The shaded area indicates the duration that antiretroviral therapy was administered. The dashed line indicates the limit of quantitation of HIV RNA. (C) Enrichment of resting CD4+ T cells from the suppressed, ES38-9-infected BLT mouse. Mononuclear cells from all tissues were pooled and subjected to magnetic negative selection of CD4+ resting T cells. Samples were analyzed by flow cytometry for the expression of CD25 and HLA-DR before (left) and after (right) negative selection. (D) Results of a viral outgrowth assay to quantify the latent reservoir. Shaded squares indicate that the well was positive for p24 in the supernatant as determined by ELISA, while nonshaded squares represent wells that were negative for p24 in the supernatant.
FIG 7
Limited variation occurs within three HLA-B*57-03-restricted Gag and Nef epitopes during HIV infection of BLT Mice. HIV-1 isolates were amplified from the thymuses and spleens of BLT humanized mice at week 16 (isolates ES38-5 and ES38-9), week 8 (isolate ES8-43), and week 4 (isolates CP4-2B and CP6-2E), and Gag and Nef epitopes were analyzed. The red and blue boxes denote two distinct but overlapping Nef epitopes, HW9 (116 to 124) and YT9 (120 to 128), respectively.
Similar articles
- Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs.
Buckheit RW 3rd, Allen TG, Alme A, Salgado M, O'Connell KA, Huculak S, Falade-Nwulia O, Williams TM, Gallant JE, Siliciano RF, Blankson JN. Buckheit RW 3rd, et al. Nat Commun. 2012 Mar 6;3:716. doi: 10.1038/ncomms1697. Nat Commun. 2012. PMID: 22395607 Free PMC article. - Effective downregulation of HLA-A*2 and HLA-B*57 by primary human immunodeficiency virus type 1 isolates cultured from elite suppressors.
Nou E, Zhou Y, Nou DD, Blankson JN. Nou E, et al. J Virol. 2009 Jul;83(13):6941-6. doi: 10.1128/JVI.00306-09. Epub 2009 Apr 22. J Virol. 2009. PMID: 19386719 Free PMC article. - Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus.
O'Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. O'Connell KA, et al. J Virol. 2010 Jul;84(14):7018-28. doi: 10.1128/JVI.00548-10. Epub 2010 May 5. J Virol. 2010. PMID: 20444904 Free PMC article. - HIV-1 replicative fitness in elite controllers.
Lobritz MA, Lassen KG, Arts EJ. Lobritz MA, et al. Curr Opin HIV AIDS. 2011 May;6(3):214-20. doi: 10.1097/COH.0b013e3283454cf5. Curr Opin HIV AIDS. 2011. PMID: 21430530 Free PMC article. Review. - [Elite controllers and the mechanisms behind spontaneous control of HIV].
Shasha D. Shasha D. Harefuah. 2013 Aug;152(8):481-5, 498. Harefuah. 2013. PMID: 24167935 Review. Hebrew.
Cited by
- A Murine Viral Outgrowth Assay to Detect Residual HIV Type 1 in Patients With Undetectable Viral Loads.
Metcalf Pate KA, Pohlmeyer CW, Walker-Sperling VE, Foote JB, Najarro KM, Cryer CG, Salgado M, Gama L, Engle EL, Shirk EN, Queen SE, Chioma S, Vermillion MS, Bullock B, Li M, Lyons CE, Adams RJ, Zink MC, Clements JE, Mankowski JL, Blankson JN. Metcalf Pate KA, et al. J Infect Dis. 2015 Nov 1;212(9):1387-96. doi: 10.1093/infdis/jiv230. Epub 2015 Apr 15. J Infect Dis. 2015. PMID: 25883388 Free PMC article. - Learning to Be Elite: Lessons From HIV-1 Controllers and Animal Models on Trained Innate Immunity and Virus Suppression.
Sugawara S, Reeves RK, Jost S. Sugawara S, et al. Front Immunol. 2022 Apr 27;13:858383. doi: 10.3389/fimmu.2022.858383. eCollection 2022. Front Immunol. 2022. PMID: 35572502 Free PMC article. Review. - A Comparison of Different Immune Activation Strategies to Reverse HIV-1 Latency.
Garliss CC, Kwaa AK, Blankson JN. Garliss CC, et al. Open Forum Infect Dis. 2020 Mar 4;7(4):ofaa082. doi: 10.1093/ofid/ofaa082. eCollection 2020 Apr. Open Forum Infect Dis. 2020. PMID: 32284948 Free PMC article. - Studies of retroviral infection in humanized mice.
Marsden MD, Zack JA. Marsden MD, et al. Virology. 2015 May;479-480:297-309. doi: 10.1016/j.virol.2015.01.017. Epub 2015 Feb 11. Virology. 2015. PMID: 25680625 Free PMC article. Review. - Validation of Variant Assembly Using HAPHPIPE with Next-Generation Sequence Data from Viruses.
Gibson KM, Steiner MC, Rentia U, Bendall ML, Pérez-Losada M, Crandall KA. Gibson KM, et al. Viruses. 2020 Jul 14;12(7):758. doi: 10.3390/v12070758. Viruses. 2020. PMID: 32674515 Free PMC article.
References
- Blankson JN. 2010. Control of HIV-1 replication in elite suppressors. Discov. Med. 9:261–266 - PubMed
- Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, Lawson VA, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan JS, Cunningham A, Dwyer D, Dowton D, Mills J. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988–991. 10.1126/science.270.5238.988 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI073146/AI/NIAID NIH HHS/United States
- R01 DA030156/DA/NIDA NIH HHS/United States
- AI080328/AI/NIAID NIH HHS/United States
- AI100775/AI/NIAID NIH HHS/United States
- F32 AI100775/AI/NIAID NIH HHS/United States
- AI096138/AI/NIAID NIH HHS/United States
- 5R01DA030156/DA/NIDA NIH HHS/United States
- U19 AI096113/AI/NIAID NIH HHS/United States
- T32 GM008752/GM/NIGMS NIH HHS/United States
- R01 AI080328/AI/NIAID NIH HHS/United States
- R56 AI080328/AI/NIAID NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- T32 GM007445/GM/NIGMS NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- R01 AI096138/AI/NIAID NIH HHS/United States
- AI073146/AI/NIAID NIH HHS/United States
- AI096113/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials