Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo - PubMed (original) (raw)
Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo
Yung-Chi Chang et al. PLoS Pathog. 2014 Jan.
Abstract
Group B Streptococcus (GBS) is a common agent of bacterial sepsis and meningitis in newborns. The GBS surface capsule contains sialic acids (Sia) that engage Sia-binding immunoglobulin-like lectins (Siglecs) on leukocytes. Here we use mice lacking Siglec-E, an inhibitory Siglec of myelomonocytic cells, to study the significance of GBS Siglec engagement during in vivo infection. We found GBS bound to Siglec-E in a Sia-specific fashion to blunt NF-κB and MAPK activation. As a consequence, Siglec-E-deficient macrophages had enhanced pro-inflammatory cytokine secretion, phagocytosis and bactericidal activity against the pathogen. Following pulmonary or low-dose intravenous GBS challenge, Siglec-E KO mice produced more pro-inflammatory cytokines and exhibited reduced GBS invasion of the central nervous system. In contrast, upon high dose lethal challenges, cytokine storm in Siglec-E KO mice was associated with accelerated mortality. We conclude that GBS Sia mimicry influences host innate immune and inflammatory responses in vivo through engagement of an inhibitory Siglec, with the ultimate outcome of the host response varying depending upon the site, stage and magnitude of infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
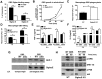
Figure 1. Augmented bactericidal activity in bone marrow-derived macrophages from Siglec-E KO mice.
(A) Wild-type Group B Streptococcus (GBS WT) showed Sia-dependent binding to mSiglec-E. GBS ΔSia mutant is an isogenic mutant lacking Sia expression. Background binding from hIgG1-immobilized wells, which served as a negative control, were subtracted from data shown. (B) Whole blood from Siglec-E KO mice showed better control of GBS growth; results pooled from two independent experiments. Siglec-E KO macrophages showed greater phagocytic activity (C) and bactericidal ability (D). GBS WT but not ΔSia mutant induced higher TNF-α (E) and IL-6 (F) secretion in macrophages from Siglec-E KO animals. Differences between different groups were calculated by two-way ANOVA with Bonferroni posttest (A–B, D–F) or unpaired t test (C). *** P<0.001; ** P<0.01; * P<0.05. (G) WT GBS stimulated macrophages recruited more SHP-1 to Siglec-E than GBS ΔSia mutant. (H) Enhanced ERK activation and IκB degradation in Siglec-E KO macrophages after GBS stimulation. Representative data from two reproducible experiments are shown.
Figure 2. Skewed cytokine responses in Siglec-E KO mice challenged in a GBS intranasal pneumonia model.
Mice were infected intranasally with 5×107 CFU WT GBS and cytokine levels in cell-free BAL fluid or lung homogenates collected 6 h post infection. (A) Bacterial load in lung tissue was calculated by dilution plating for CFU enumeration. (B) Cells from BAL were counted and stained with mAb to F4/80 and Gr-1 to quantitate infiltrating cell populations. IL-1β (C) and IL-6 (D) in the BAL and IL-10 in lung homogenates (E) were examined by ELISA analysis. Data shown are means ± SEM and each circle denotes 1 mouse (n = 8 for WT and n = 7 for mSiglec-E KO mice). The difference between different groups was calculated by Mann-Whitney test.
Figure 3. Absence of Siglec-E exacerbates inflammation and accelerates mortality in a systemic lethal dose GBS challenge.
WT and Siglec-E KO mice were challenged intravenously with 3×108 CFU of WT GBS. (A) Kaplan-Meier plot is shown for survival analysis (n = 10 for each group). Blood and brains were collected 18 h after GBS infection to measure IL-6 (B), serum amyloid A (C) and bacteria loads (D and E). Data shown are means ± SEM and each circle denotes 1 mouse (n = 6 for WT and n = 8 for Siglec-E KO mice). Differences between different groups were calculated by Mann-Whitney test (B–E).
Figure 4. Reduced brain dissemination and enhanced bactericidal responses in Siglec-E deficient mice upon sublethal GBS challenge.
Comparison of bacterial counts (expressed in CFU) recovered from the kidney (A) and brain (B) of WT mice and Siglec-E KO mice 48 h after intravenous challenge with 1×108 CFU of WT GBS. (C) Brain bacterial counts were corrected for blood contamination (brain/blood ratio) using a conservative estimate of the mouse cerebral blood volume (2.5 ml per 100 g tissue). mRNA expression of IL-1β (D) and IL-12 (E) in lung and IL-10 in spleen (F) was examined by quantitative real time RT-PCR analysis. Results pooled the data from two independent experiment with final numbers of n = 14 for each group. Each circle denotes 1 mouse (A–F). Siglec-E KO microglia cells showed greater bactericidal ability (G) and produced higher levels of TNF-α (H) after GBS challenge. Statistical analysis was performed by Mann-Whitney test (A–F), two-way ANOVA with Bonferroni posttest (G) and one-way ANOVA with Tukey's multiple comparison test (H). *P<0.05.
Similar articles
- Sialic Acid-Siglec-E Interactions Regulate the Response of Neonatal Macrophages to Group B Streptococcus.
Lund SJ, Del Rosario PGB, Honda A, Caoili KJ, Hoeksema MA, Nizet V, Patras KA, Prince LS. Lund SJ, et al. Immunohorizons. 2024 May 1;8(5):384-396. doi: 10.4049/immunohorizons.2300076. Immunohorizons. 2024. PMID: 38809232 Free PMC article. - Dual actions of group B Streptococcus capsular sialic acid provide resistance to platelet-mediated antimicrobial killing.
Uchiyama S, Sun J, Fukahori K, Ando N, Wu M, Schwarz F, Siddiqui SS, Varki A, Marth JD, Nizet V. Uchiyama S, et al. Proc Natl Acad Sci U S A. 2019 Apr 9;116(15):7465-7470. doi: 10.1073/pnas.1815572116. Epub 2019 Mar 25. Proc Natl Acad Sci U S A. 2019. PMID: 30910970 Free PMC article. - Sialic Acid-Siglec-E Interactions During Pseudomonas aeruginosa Infection of Macrophages Interferes With Phagosome Maturation by Altering Intracellular Calcium Concentrations.
Mukherjee K, Khatua B, Mandal C. Mukherjee K, et al. Front Immunol. 2020 Feb 28;11:332. doi: 10.3389/fimmu.2020.00332. eCollection 2020. Front Immunol. 2020. PMID: 32184783 Free PMC article. - Siglecs at the Host-Pathogen Interface.
Chang YC, Nizet V. Chang YC, et al. Adv Exp Med Biol. 2020;1204:197-214. doi: 10.1007/978-981-15-1580-4_8. Adv Exp Med Biol. 2020. PMID: 32152948 Free PMC article. Review. - The interplay between Siglecs and sialylated pathogens.
Chang YC, Nizet V. Chang YC, et al. Glycobiology. 2014 Sep;24(9):818-25. doi: 10.1093/glycob/cwu067. Epub 2014 Jul 4. Glycobiology. 2014. PMID: 24996821 Free PMC article. Review.
Cited by
- Sialic Acid-Siglec-E Interactions Regulate the Response of Neonatal Macrophages to Group B Streptococcus.
Lund SJ, Del Rosario PGB, Honda A, Caoili KJ, Hoeksema MA, Nizet V, Patras KA, Prince LS. Lund SJ, et al. Immunohorizons. 2024 May 1;8(5):384-396. doi: 10.4049/immunohorizons.2300076. Immunohorizons. 2024. PMID: 38809232 Free PMC article. - Distinct Group B Streptococcus Sequence and Capsule Types Differentially Impact Macrophage Stress and Inflammatory Signaling Responses.
Flaherty RA, Aronoff DM, Gaddy JA, Petroff MG, Manning SD. Flaherty RA, et al. Infect Immun. 2021 Apr 16;89(5):e00647-20. doi: 10.1128/IAI.00647-20. Print 2021 Apr 16. Infect Immun. 2021. PMID: 33558317 Free PMC article. - Progressive Control of Streptococcus agalactiae-Induced Innate Inflammatory Response Is Associated with Time Course Expression of MicroRNA-223 by Neutrophils.
Deny M, Romano M, Denis O, Casimir G, Chamekh M. Deny M, et al. Infect Immun. 2020 Nov 16;88(12):e00563-20. doi: 10.1128/IAI.00563-20. Print 2020 Nov 16. Infect Immun. 2020. PMID: 32958526 Free PMC article. - Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses.
Läubli H, Varki A. Läubli H, et al. Cell Mol Life Sci. 2020 Feb;77(4):593-605. doi: 10.1007/s00018-019-03288-x. Epub 2019 Sep 4. Cell Mol Life Sci. 2020. PMID: 31485715 Free PMC article. Review. - Discovery, classification, evolution and diversity of Siglecs.
Angata T, Varki A. Angata T, et al. Mol Aspects Med. 2023 Apr;90:101117. doi: 10.1016/j.mam.2022.101117. Epub 2022 Aug 18. Mol Aspects Med. 2023. PMID: 35989204 Free PMC article. Review.
References
- Edwards MS (2006) Issues of antimicrobial resistance in group B streptococcus in the era of intrapartum antibiotic prophylaxis. Semin Pediatr Infect Dis 17: 149–152. - PubMed
- Heath PT, Schuchat A (2007) Perinatal group B streptococcal disease. Best Pract Res Clin Obstet Gynaecol 21: 411–424. - PubMed
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, et al. (2011) Bacterial meningitis in the United States, 1998–2007. N Engl J Med 364: 2016–2025. - PubMed
- Ferrieri P, Cleary PP, Seeds AE (1977) Epidemiology of group-B streptococcal carriage in pregnant women and newborn infants. J Med Microbiol 10: 103–114. - PubMed
- Galask RP, Varner MW, Petzold CR, Wilbur SL (1984) Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol 148: 915–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL107150/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- HL057345/HL/NHLBI NIH HHS/United States
- R01 CA038701/CA/NCI NIH HHS/United States
- Wellcome Trust/United Kingdom
- P01 HL107150/HL/NHLBI NIH HHS/United States
- 081882/Wellcome Trust/United Kingdom
- HD051796/HD/NICHD NIH HHS/United States
- R01 HD051796/HD/NICHD NIH HHS/United States
- P01 HL057345/HL/NHLBI NIH HHS/United States
- T35 HL007491/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials