Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection - PubMed (original) (raw)
. 2014 Jan;10(1):e1003856.
doi: 10.1371/journal.ppat.1003856. Epub 2014 Jan 2.
Ejuan Zhang 1, Zhiyong Ma 1, Weimin Wu 1, Anna Kosinska 1, Xiaoyong Zhang 2, Inga Möller 1, Pia Seiz 3, Dieter Glebe 3, Baoju Wang 4, Dongliang Yang 4, Mengji Lu 1, Michael Roggendorf 1
Affiliations
- PMID: 24391505
- PMCID: PMC3879364
- DOI: 10.1371/journal.ppat.1003856
Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection
Jia Liu et al. PLoS Pathog. 2014 Jan.
Abstract
Hepatitis B virus (HBV) persistence is facilitated by exhaustion of CD8 T cells that express the inhibitory receptor programmed cell death-1 (PD-1). Improvement of the HBV-specific T cell function has been obtained in vitro by inhibiting the PD-1/PD-ligand 1 (PD-L1) interaction. In this study, we examined whether in vivo blockade of the PD-1 pathway enhances virus-specific T cell immunity and leads to the resolution of chronic hepadnaviral infection in the woodchuck model. The woodchuck PD-1 was first cloned, characterized, and its expression patterns on T cells from woodchucks with acute or chronic woodchuck hepatitis virus (WHV) infection were investigated. Woodchucks chronically infected with WHV received a combination therapy with nucleoside analogue entecavir (ETV), therapeutic DNA vaccination and woodchuck PD-L1 antibody treatment. The gain of T cell function and the suppression of WHV replication by this therapy were evaluated. We could show that PD-1 expression on CD8 T cells was correlated with WHV viral loads during WHV infection. ETV treatment significantly decreased PD-1 expression on CD8 T cells in chronic carriers. In vivo blockade of PD-1/PD-L1 pathway on CD8 T cells, in combination with ETV treatment and DNA vaccination, potently enhanced the function of virus-specific T cells. Moreover, the combination therapy potently suppressed WHV replication, leading to sustained immunological control of viral infection, anti-WHs antibody development and complete viral clearance in some woodchucks. Our results provide a new approach to improve T cell function in chronic hepatitis B infection, which may be used to design new immunotherapeutic strategies in patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
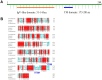
Figure 1. Analysis of the deduced amino acid sequence of woodchuck PD-1.
(A) Predicted secondary structure of woodchuck PD-1. The locations of immunoglobulin V-like domain (Ig-V-like domain) and transmembrane region (TM domain) are indicated. (B) Alignment of woodchuck PD-1 with human PD-1 and mouse PD-1. Consensus sequences are marked with red. Blue boxes indicate the conserved ITIM and ITSM motifs.
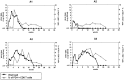
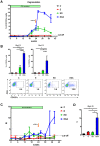
Figure 2. Kinetics of PD-1 expression on CD8 T cells during acute WHV infection.
Four adult woodchucks were inoculated with 1×107 or 1×109 WHV genome equivalents. Sera and PBMCs of infected woodchucks were freshly isolated at indicated time points. WHV viral loads (black dots) were quantified by real-time PCR, and percentages of PD-1+ CD8 (CD3+ CD4−) T cells (gray squares) were analyzed by FACS. LOD: lower limit of detection.
Figure 3. Expression of PD-1 on PBMCs and T cells during chronic WHV infection.
(A) PBMCs were isolated from naïve (n = 4), WHV infection resolved (n = 4), and untreated chronically WHV infected woodchucks (n = 7). The absolute copy numbers of PD-1 mRNA of total PBMCs were analyzed by real-time PCR. The percentages of PD-1+ CD8 (CD3+ CD4−) T cells were analyzed by FACS. (B) The mean fluorescence intensity (MFI) of PD-1 expression on CD8 T cells was calculated and compared between different groups. Representative dot plot shows the population of PD-1hi CD8 T cells of chronically WHV infected woodchucks. (C) PD-1 expression in chronically WHV infected woodchucks before and 6 weeks after ETV treatment (n = 5) were compared. Left: The absolute copy numbers of PD-1 mRNA of total PBMCs were analyzed by real-time PCR. Right: The percentages of PD-1+ CD8 T cells (black dots) are coordinated with the WHV viral loads (gray columns) in ETV treated woodchucks. (D) The changes of mean fluorescence intensity (MFI) of PD-1 expression on CD8 T cells before and 6 weeks after ETV treatment were compared. Representative dot plot shows the disappearance of PD-1hi CD8 T cells in chronically WHV infected woodchucks after ETV treatment. (E) The percentages of PD-1+ CD4 T cells (CD3+ CD4+) were analyzed by FACS and compared to that of CD8 T cells (CD3+ CD4−). Representative dot plot shows the populations of PD-1+ CD4 T cells and PD-1+ CD8 T cells of chronically WHV infected woodchucks. (F) The percentages of PD-1+ CD4 T cells of chronically WHV infected woodchucks before and after ETV treatment (n = 5) were compared.
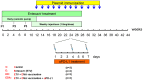
Figure 4. Schema of triple combination therapy of ETV treatment, DNA vaccination and in vivo PD-L1 blockade.
The antiviral drug ETV was administered for 28 weeks to suppress the WHV replication. Initially, the drug was administered for 12 weeks by using osmotic pumps which were implanted surgically under the skin of the animals. Pumps were exchanged every 4 weeks and overall 3 pumps were implanted for each woodchuck. P1, P2 and P3 mean the timings of the pump implantations. From week 10 to 28, subcutaneous injections of 1.5-1/PD-L1 pathway blockade, woodchucks were treated with rabbit polyclonal PD-L1 blocking antibody (αPD-L1) 3 times in week 24. Four groups of woodchucks (n = 3/group) were included. C: control group without any treatment; E: ETV treated only group; ED: ETV in combination with DNA vaccinations group; EDA: ETV and DNA vaccination in combination with anti-PDL1 antibody treatment group.
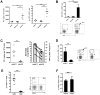
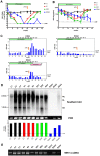
Figure 5. In vivo PD-L1 blockade synergizes with therapeutic vaccination to enhance WHV-specific T cell immunity.
(A) WHcAg-specific T cell responses of differently treated woodchucks were analyzed by CD107a degranulation assay. The kinetics of WHcAg-specific CD8 T cell response of 4 differently treated groups of woodchucks is presented. C: control group without any treatment; E: ETV treated only group; ED: ETV in combination with DNA vaccinations; EDA: ETV and DNA vaccination in combination with anti-PDL1 antibody treatment. (B) Upper: Comparison of the strength of WHcAg-specific T cell response of woodchucks with different treatments at week 25 and week 38. Lower: Representative dot plots show the population of CD107a+ CD8 T cells of treated woodchuck PBMCs in response to WHcAg-specific peptide stimulation (week 25). (C) WHcAg-specific T cell responses of differently treated woodchucks were analyzed by proliferation assay. The kinetics of PBMCs proliferation in response to WHV core protein stimulation of woodchucks with different treatments is presented. (D) Comparison of the strength of PBMCs proliferation of woodchucks with different treatments at week 38. Stimulation index (SI) is calculated with the formula: (stimulated cpm - blank cpm)/(unstimulated cpm - blank cpm).
Figure 6. In vivo PD-L1 blockade synergizes with therapeutic vaccination to control WHV replication.
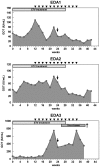
Serum WHV DNA (A) and WHsAg (B) concentrations of different treatment groups are presented at indicated time points. C: control group without any treatment; E: ETV treated only group; ED: ETV in combination with DNA vaccinations; EDA: ETV and DNA vaccination in combination with anti-PDL1 antibody treatment. LOD: lower limit of detection. (C) The kinetics of serum anti-WHs antibodies levels of woodchucks with triple combination treatment is presented. Anti-WHs antibodies level was determined using the following formula: S/N ratio = sample OD value/negative control OD value. Cut-off value of S/N ratio was 2.1. (D) Detection of WHV replication intermediates in liver tissues of differently treated woodchucks. Liver samples were taken 14 weeks after cessation of ETV treatment. Total DNA were extracted from the liver samples and subjected to Southern blotting (upper), PCR (middle), and realtime PCR (lower). RC: relaxed circular DNA, DL: double stranded linear DNA, SS: single stranded DNA. (E) Detection of WHV cccDNA in liver tissues of differently treated woodchucks. Extracted liver DNA samples were digested with Plasmid-Safe ATP-Dependent DNase and subjected cccDNA-specific PCR with primers amplifying the WHV gap-spanning region.
Figure 7. Liver transaminase GOT levels of woodchucks with combination treatment.
The GOT values (gray areas) of the woodchuck sera were measured up to the end of the observation period. The duration of ETV treatment is indicated with a gray box, DNA vaccinations are indicated with black triangles, and αPD-L1 treatment is indicated with a black arrow. WHcAg-specific CD8 T cell responses of the woodchucks are indicated with black bars.
Similar articles
- Combination of DNA prime--adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model.
Kosinska AD, Zhang E, Johrden L, Liu J, Seiz PL, Zhang X, Ma Z, Kemper T, Fiedler M, Glebe D, Wildner O, Dittmer U, Lu M, Roggendorf M. Kosinska AD, et al. PLoS Pathog. 2013;9(6):e1003391. doi: 10.1371/journal.ppat.1003391. Epub 2013 Jun 13. PLoS Pathog. 2013. PMID: 23785279 Free PMC article. - The expression of PD-1 ligands and their involvement in regulation of T cell functions in acute and chronic woodchuck hepatitis virus infection.
Zhang E, Zhang X, Liu J, Wang B, Tian Y, Kosinska AD, Ma Z, Xu Y, Dittmer U, Roggendorf M, Yang D, Lu M. Zhang E, et al. PLoS One. 2011;6(10):e26196. doi: 10.1371/journal.pone.0026196. Epub 2011 Oct 14. PLoS One. 2011. PMID: 22022563 Free PMC article. - The Woodchuck, a Nonprimate Model for Immunopathogenesis and Therapeutic Immunomodulation in Chronic Hepatitis B Virus Infection.
Roggendorf M, Kosinska AD, Liu J, Lu M. Roggendorf M, et al. Cold Spring Harb Perspect Med. 2015 Oct 28;5(12):a021451. doi: 10.1101/cshperspect.a021451. Cold Spring Harb Perspect Med. 2015. PMID: 26511761 Free PMC article. Review. - Safety and efficacy of anti-PD-L1 therapy in the woodchuck model of HBV infection.
Balsitis S, Gali V, Mason PJ, Chaniewski S, Levine SM, Wichroski MJ, Feulner M, Song Y, Granaldi K, Loy JK, Thompson CM, Lesniak JA, Brockus C, Kishnani N, Menne S, Cockett MI, Iyer R, Mason SW, Tenney DJ. Balsitis S, et al. PLoS One. 2018 Feb 14;13(2):e0190058. doi: 10.1371/journal.pone.0190058. eCollection 2018. PLoS One. 2018. PMID: 29444087 Free PMC article. - The woodchuck: a model for therapeutic vaccination against hepadnaviral infection.
Roggendorf M, Yang D, Lu M. Roggendorf M, et al. Pathol Biol (Paris). 2010 Aug;58(4):308-14. doi: 10.1016/j.patbio.2010.04.005. Epub 2010 Jun 19. Pathol Biol (Paris). 2010. PMID: 20646874 Review.
Cited by
- Toll-like receptor (TLR)-mediated innate immune responses in the control of hepatitis B virus (HBV) infection.
Zhang E, Lu M. Zhang E, et al. Med Microbiol Immunol. 2015 Feb;204(1):11-20. doi: 10.1007/s00430-014-0370-1. Epub 2014 Dec 31. Med Microbiol Immunol. 2015. PMID: 25550115 Free PMC article. Review. - Discontinuation of Nucleos(t)ide Analogues in HBeAg Negative Chronic Hepatitis B Patients: Risks and Benefits.
Korkmaz P, Demirtürk N. Korkmaz P, et al. Infect Dis Clin Microbiol. 2024 Jun 28;6(2):70-77. doi: 10.36519/idcm.2024.339. eCollection 2024 Jun. Infect Dis Clin Microbiol. 2024. PMID: 39005698 Free PMC article. Review. - Combination Treatment with the Vimentin-Targeting Antibody hzVSF and Tenofovir Suppresses Woodchuck Hepatitis Virus Infection in Woodchucks.
Korolowicz KE, Suresh M, Li B, Huang X, Yon C, Kallakury BV, Lee KP, Park S, Kim YW, Menne S. Korolowicz KE, et al. Cells. 2021 Sep 5;10(9):2321. doi: 10.3390/cells10092321. Cells. 2021. PMID: 34571970 Free PMC article. - Genetic variants of programmed cell death 1 are associated with HBV infection and liver disease progression.
Hoan NX, Huyen PTM, Binh MT, Trung NT, Giang DP, Linh BT, Dung DTN, Pallerla SR, Kremsner PG, Velavan TP, Bang MH, Song LH. Hoan NX, et al. Sci Rep. 2021 Apr 8;11(1):7772. doi: 10.1038/s41598-021-87537-9. Sci Rep. 2021. PMID: 33833369 Free PMC article. - Therapeutic vaccination for treatment of chronic hepatitis B.
Cargill T, Barnes E. Cargill T, et al. Clin Exp Immunol. 2021 Aug;205(2):106-118. doi: 10.1111/cei.13614. Epub 2021 Jun 8. Clin Exp Immunol. 2021. PMID: 33969474 Free PMC article. Review.
References
- Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, et al. (2005) Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 365: 123–129. - PubMed
- Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, et al. (2005) Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 352: 2682–2695. - PubMed
- Locarnini S, Mason WS (2006) Cellular and virological mechanisms of HBV drug resistance. J Hepatol 44: 422–431. - PubMed
- Zoulim F, Locarnini S (2009) Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology 137: 1593–1608 e1591–1592. - PubMed
- Klenerman P, Hill A (2005) T cells and viral persistence: lessons from diverse infections. Nat Immunol 6: 873–879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by the Deutsche Forschungsgemeinschaft (TRR60 and GK 1045/2), National Major Science and Technology Project for Infectious Diseases of China (2008ZX10002-011, 2012ZX10004503), the National Natural Science Foundation of China (No. 30271170, 30571646, 81101248), and the International Science & Technology Cooperation Program of China (2011DFA31030). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials