Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia - PubMed (original) (raw)
Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia
Tiziana Cotechini et al. J Exp Med. 2014.
Abstract
Fetal growth restriction (FGR) and preeclampsia (PE) are often associated with abnormal maternal inflammation, deficient spiral artery (SA) remodeling, and altered uteroplacental perfusion. Here, we provide evidence of a novel mechanistic link between abnormal maternal inflammation and the development of FGR with features of PE. Using a model in which pregnant rats are administered low-dose lipopolysaccharide (LPS) on gestational days 13.5-16.5, we show that abnormal inflammation resulted in FGR mediated by tumor necrosis factor-α (TNF). Inflammation was also associated with deficient trophoblast invasion and SA remodeling, as well as with altered uteroplacental hemodynamics and placental nitrosative stress. Moreover, inflammation increased maternal mean arterial pressure (MAP) and was associated with renal structural alterations and proteinuria characteristic of PE. Finally, transdermal administration of the nitric oxide (NO) mimetic glyceryl trinitrate prevented altered uteroplacental perfusion, LPS-induced inflammation, placental nitrosative stress, renal structural and functional alterations, increase in MAP, and FGR. These findings demonstrate that maternal inflammation can lead to severe pregnancy complications via a mechanism that involves increased maternal levels of TNF. Our study provides a rationale for the use of antiinflammatory agents or NO-mimetics in the treatment and/or prevention of inflammation-associated pregnancy complications.
Figures
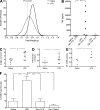
Figure 1.
LPS administration results in TNF-mediated FGR. Analysis of fetal weights on GD 17.5 after saline (n = 305 fetuses from 22 dams) or LPS (n = 258 fetuses from 28 dams) administration on GD 13.5–16.5 (A). Plasma TNF levels measured 2 h after saline or LPS administration on GD 13.5, and 2 h after LPS administration on GD 15.5 (B). White blood cell counts (C) and number of circulating monocytes assessed on GD 17.5 (D). Presence of activated macrophages (CD68+) in MT of uteroplacental units from LPS- or saline-treated rats on GD 17.5 (E). Effect of Eta (administered on GD 13.5 and 15.5) on LPS-induced FGR measured on GD 17.5 (F). Eta + LPS n = 174 fetuses from 18 dams; Eta + saline n = 88 fetuses from 6 dams). *, P < 0.05; **, P < 0.01; ****, P < 0.0001. Bar graphs represent mean ± SEM.
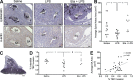
Figure 2.
TNF is causally linked to deficient trophoblast invasion and SA remodeling associated with FGR. Spiral arteries from all treatment groups exhibited evidence of remodeling, including the presence of cytokeratin-positive endovascular trophoblast cells (black cells) resting on a fibrinoid layer (arrows) and the absence of α-actin+ (brown stain) smooth muscle (A). Effect of LPS administration on SA remodeling (A and B). Mean SA cross-sectional area in uteroplacental units (B). Interstitial trophoblast invasion into the MT was quantified as the percent area of the MT infiltrated by cytokeratin-positive trophoblast cells (C and D). Correlation of interstitial trophoblast invasion and mean spiral artery cross-sectional area for all treatment groups (E). n = 1–2 implantation sites for 5–9 animals/group; MT, mesometrial triangle; P, placenta; TBC, trophoblast cells. Φ, P = 0.0530; * P < 0.05. Bars: (A) 50 µm; (C) 1 mm.
Figure 3.
LPS-induced FGR is linked to uteroplacental hemodynamic alterations associated with nitrosative stress. Ultrasound biomicroscopy was used to visualize the vasculature within implantation sites, including spiral arteries, maternal channels, and the corresponding fetal umbilical artery (A). Mean SA RI measured from implantation sites (2–3 spiral arteries measured from 3–4 implantation sites per animal; n = 6–7 dams per group; B). Correlation of mean SA RI with the corresponding umbilical artery PSV from LPS-treated animals (C). Correlation of mean SA RI with the corresponding maternal channel RI across all treatment groups (D). Detectable nitrotyrosine (shaded box) in placentas from rats treated with saline, LPS, or LPS + GTN (E). *, P < 0.05; **, P < 0.01. UmbA, umbilical artery; MC, maternal channel; MT, mesometrial triangle; P, placenta; SA, spiral artery. Whiskers in B represent the 5th and 95th percentiles.
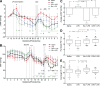
Figure 4.
Elevated MAP is causally linked to increased maternal TNF levels and is associated with FGR at birth. To observe the treatment-specific effects on MAP, all animals were normalized to GD 11. Change in MAP corresponding to saline (n = 5 dams) or LPS (n = 5 dams) administration on GD 13.5–16.5 (dashed vertical lines) and Eta (n = 5) or GTN administration (n = 5) to LPS-treated animals (A). Full gestational MAP profiles (GD 0–PD 7) in rats from all treatment groups (n = 5 for each group; B). Mean overall change in MAP spanning GD 11–PD 7 in rats from all treatment groups (C). Weight of pups assessed on PD 1.5 (D) and 7.5 (E); saline, n = 45 pups; LPS, n = 35 pups; Eta + LPS, n = 56 pups; GTN + LPS, n = 53 pups. Φ, P < 0.1; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Symbols in A denote significant differences from LPS-treated animals; asterisks and asterisks within brackets in B denote significant differences at each day compared with baseline (GD 11); shaded regions in A and B represent the day of birth for all dams; points in A and C represent mean ± SEM; points in B represent 24-h mean MAP ± SEM; whiskers in D and E represent the 10th and 90th percentiles.
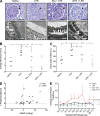
Figure 5.
LPS-induced renal alterations and proteinuria are causally linked to TNF and can be prevented by GTN. Glomerular pathology of maternal kidneys from LPS-treated animals including hypercellularity, occlusion of the urinary space (arrows), and thickening of the GBM (yellow asterisks; A). Degree of glomerular pathology (mean of 20 glomeruli/animal; n = 5–6 animals/group; B) and assessment of GBM thickening across all treatment groups (C). Correlation between the change in MAP and change in urinary protein/creatinine from LPS-treated dams (D). Mean protein/creatinine ratio from urine collected on GD 10.5–16.5. 17.5, 19.5 and PD 7.5 across all treatment groups (E). The curves in E were generated using the mean ± SEM protein/creatinine ratios from urine collected from 3–5 rats/treatment group over the indicated GDs. Because of missing values and variability in the baseline protein/creatinine ratios before initiation of treatments, statistical analysis in (E) was performed on the changes in protein/creatinine ratios rather than on the actual raw values using ANOVA followed by Bonferroni post-hoc test. Φ, P = 0.0562; *, P < 0.05; **, P < 0.01. Bars: (top row) 50 µm; (bottom row) 500 nm.
Similar articles
- Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat.
Barrientos G, Pussetto M, Rose M, Staff AC, Blois SM, Toblli JE. Barrientos G, et al. Mol Hum Reprod. 2017 Jul 1;23(7):509-519. doi: 10.1093/molehr/gax024. Mol Hum Reprod. 2017. PMID: 28402512 - Inflammation-induced fetal growth restriction in rats is associated with increased placental HIF-1α accumulation.
Robb KP, Cotechini T, Allaire C, Sperou A, Graham CH. Robb KP, et al. PLoS One. 2017 Apr 19;12(4):e0175805. doi: 10.1371/journal.pone.0175805. eCollection 2017. PLoS One. 2017. PMID: 28423052 Free PMC article. - Aberrant maternal inflammation as a cause of pregnancy complications: A potential therapeutic target?
Cotechini T, Graham CH. Cotechini T, et al. Placenta. 2015 Aug;36(8):960-6. doi: 10.1016/j.placenta.2015.05.016. Epub 2015 Jun 4. Placenta. 2015. PMID: 26094029 Review. - Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome.
Lyall F, Robson SC, Bulmer JN. Lyall F, et al. Hypertension. 2013 Dec;62(6):1046-54. doi: 10.1161/HYPERTENSIONAHA.113.01892. Epub 2013 Sep 23. Hypertension. 2013. PMID: 24060885 - Pathophysiology of placental-derived fetal growth restriction.
Burton GJ, Jauniaux E. Burton GJ, et al. Am J Obstet Gynecol. 2018 Feb;218(2S):S745-S761. doi: 10.1016/j.ajog.2017.11.577. Am J Obstet Gynecol. 2018. PMID: 29422210 Review.
Cited by
- Organoids as Novel Models for Embryo Implantation Study.
Wei Y, Zhang C, Fan G, Meng L. Wei Y, et al. Reprod Sci. 2021 Jun;28(6):1637-1643. doi: 10.1007/s43032-021-00501-w. Epub 2021 Mar 1. Reprod Sci. 2021. PMID: 33650092 Review. - Single administration of ultra-low-dose lipopolysaccharide in rat early pregnancy induces TLR4 activation in the placenta contributing to preeclampsia.
Xue P, Zheng M, Gong P, Lin C, Zhou J, Li Y, Shen L, Diao Z, Yan G, Sun H, Hu Y. Xue P, et al. PLoS One. 2015 Apr 8;10(4):e0124001. doi: 10.1371/journal.pone.0124001. eCollection 2015. PLoS One. 2015. PMID: 25853857 Free PMC article. - Fetal growth restriction is a host specific response to infection with an impaired spiral artery remodeling-inducing strain of Porphyromonas gingivalis.
Tavarna T, Phillips PL, Wu XJ, Reyes L. Tavarna T, et al. Sci Rep. 2020 Sep 3;10(1):14606. doi: 10.1038/s41598-020-71762-9. Sci Rep. 2020. PMID: 32884071 Free PMC article. - The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction.
Alfian I, Chakraborty A, Yong HEJ, Saini S, Lau RWK, Kalionis B, Dimitriadis E, Alfaidy N, Ricardo SD, Samuel CS, Murthi P. Alfian I, et al. Cells. 2022 Apr 21;11(9):1413. doi: 10.3390/cells11091413. Cells. 2022. PMID: 35563719 Free PMC article. - Utero-placental vascular remodeling during late gestation in Sprague-Dawley rats.
Spradley FT, Ge Y, Granger JP, Chade AR. Spradley FT, et al. Pregnancy Hypertens. 2020 Apr;20:36-43. doi: 10.1016/j.preghy.2020.02.007. Epub 2020 Mar 3. Pregnancy Hypertens. 2020. PMID: 32172168 Free PMC article.
References
- Acharya G., Wilsgaard T., Berntsen G.K., Maltau J.M., Kiserud T. 2005. Reference ranges for serial measurements of blood velocity and pulsatility index at the intra-abdominal portion, and fetal and placental ends of the umbilical artery. Ultrasound Obstet. Gynecol. 26:162–169 10.1002/uog.1902 - DOI - PubMed
- Bauer S., Pollheimer J., Hartmann J., Husslein P., Aplin J.D., Knöfler M. 2004. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J. Clin. Endocrinol. Metab. 89:812–822 10.1210/jc.2003-031351 - DOI - PubMed
- Berks D., Steegers E.A., Molas M., Visser W. 2009. Resolution of hypertension and proteinuria after preeclampsia. Obstet. Gynecol. 114:1307–1314 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous