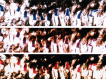Filtering and polychromatic vision in mantis shrimps: themes in visible and ultraviolet vision - PubMed (original) (raw)
Review
Filtering and polychromatic vision in mantis shrimps: themes in visible and ultraviolet vision
Thomas W Cronin et al. Philos Trans R Soc Lond B Biol Sci. 2014.
Abstract
Stomatopod crustaceans have the most complex and diverse assortment of retinal photoreceptors of any animals, with 16 functional classes. The receptor classes are subdivided into sets responsible for ultraviolet vision, spatial vision, colour vision and polarization vision. Many of these receptor classes are spectrally tuned by filtering pigments located in photoreceptors or overlying optical elements. At visible wavelengths, carotenoproteins or similar substances are packed into vesicles used either as serial, intrarhabdomal filters or lateral filters. A single retina may contain a diversity of these filtering pigments paired with specific photoreceptors, and the pigments used vary between and within species both taxonomically and ecologically. Ultraviolet-filtering pigments in the crystalline cones serve to tune ultraviolet vision in these animals as well, and some ultraviolet receptors themselves act as birefringent filters to enable circular polarization vision. Stomatopods have reached an evolutionary extreme in their use of filter mechanisms to tune photoreception to habitat and behaviour, allowing them to extend the spectral range of their vision both deeper into the ultraviolet and further into the red.
Keywords: Stomatopod; colour vision; polarization vision; spectral filtering; ultraviolet vision; visual ecology.
Figures
Figure 1.
Compound eyes of stomatopod crustaceans assigned to three different superfamilies, all containing six-row midbands. (a) Hemisquilla californiensis (Hemisquilloidea). The eye is elongated, and the midband is fairly prominent. (b) Lysiosquillina sulcata (Lysiosquilloidea). This eye is very tall, and the midband is not very prominent. The dark spots on the eyes are the pseudo-pupils; the animal's right eye is directed at the camera so that all three pseudo-pupils are visible. (c) Odontodactylus scyllarus (Gonodactyloidea). The eye is nearly spherical, with a very prominent midband. Here, the left eye is pointed in the camera's direction and shows three pseudo-pupils. (Online version in colour.)
Figure 2.
(a) A diagrammatic view of the retina of Odontodactylus scyllarus. The crystalline cones are to the right, and the ommatidia of the dorsal half of the eye, the ventral half, and the midband are indicated. R8 rhabdomeres are shaded, and main rhabdoms are hatched. The tiers of the first through the fourth rows are indicated by diagonal hatching; untiered main rhabdoms are crosshatched. The black segments, indicated by closed arrowheads, are the intrarhabdomal filters. Open arrowheads indicate the R8 rhabdomeres in the two most ventral ommatidial rows, which serve as polarization filters. Circles indicate nuclei. (b) Normalized absorbance spectra of visual pigments in main rhabdoms of O. scyllarus, based on between nine and 20 photobleaches per class. Jagged lines show original data, whereas smooth lines are best-fit template spectra. The _λ_max of each fitted spectrum is given in the panel; D, distal tier; P, proximal tier. Note that in the tiered rows, one through four, the distal visual pigment always has a shorter absorption maximum than the proximal pigment.
Figure 3.
(a) Normalized absorbance spectra of filter pigments in the third and fourth midband rows of Odontodactylus scyllarus. The distal filters are plotted as thin lines and the proximal filters as thick ones. Row 2 filters (which are identical at the two locations) are plotted in black, and row 3 filters in grey. (b) Computed normalized sensitivity spectra of photoreceptors in main rhabdoms of O. scyllarus, using lengths of each receptor class with a visual pigment density of 0.008 per micrometre together with total filter absorbance for functions in the second and third midband rows. Note that the tiered rows contain pairs of narrow spectral sensitivities, with the distal tier (D) placed to shorter wavelengths than the proximal tier (P). The distal tiers of the first and fourth midband rows are probably narrowed by absorption in the R8 rhabdomeres, but the actual values are not available for these calculations. Sensitivity functions in the untiered rhabdoms are broad due to self-screening by visual pigments in long rhabdoms.
Figure 4.
(a–d) Photographs of intrarhabdomal filters and lateral filtering pigments in cryosections of fresh retinas of various stomatopod crustaceans. (a) Longitudinal section showing proximal filters in second row (yellow) and third row (red) ommatidia of Odontodactylus scyllarus. Note the dense coloration of the filters and their relatively extended lengths. The clear sections above and below the filters are the rhabdoms. (b) Blue-coloured filters in third row ommatidia of a species of Gonodactylus. (c) A cross section through the midband of the lysiosquilloid stomatopod C. scolopendra, showing the six rows with the dorsal-most row to the left. Yellow intrarhabdomal filters are visible in the second row, and a variety of coloured lateral filtering pigments can be seen, especially the red lateral filters in the third row and golden ones in the fifth and sixth rows. (d) Longitudinal section of the distal filter and associated lateral screening pigment at the junction of the distal tier and R8 rhabdomere in the third midband row. Note that the lateral screen extends down along the distal tier, acting together with the intrarhabdomal filter to tune the associated receptor. The colour of the screening pigment resembles that of the pigment in the filter. (e) An electron micrograph of the distal filter in a second-row ommatidium of Neogonodactylus oerstedii, showing the four-part structure (from the four retinular cells contributing to the receptor at that level) and the collection of vesicles, presumably containing the coloured pigment, in the filter.
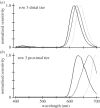
Figure 5.
Normalized absorbance spectra taken in cryosections of third-row, proximal intrarhabdomal filters. (a,b) Scans from filters in retinas of two individuals of Gonodactylopsis spongicola, one collected at a depth of 5 m and the other at 15 m, showing the differences between the two sample sets and the variability within a single filter class in a single retina. (c) Successive scans of a single filter (from Neogonodactylus oerstedii) taken after various times of warming the sample on a hotplate. The spectrum of the original, blue-coloured filter (e.g. figure 4_b_) is the rightmost scan; the final scan of a red-appearing filter is to the left. Note that these spectra are quite similar to those from the untreated cryosections from G. spongicola.
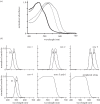
Figure 6.
(a,b) Normalized sensitivity spectra of distal and proximal tier rhabdoms from the third ommatidial row of midbands of individual Gonodactylopsis spongicola collected at various depths from near the surface to approximately 32 m depth. The light grey curve is from the shallow-living individual, the mid-grey grey curve for an animal living at moderate depth, and the black curve for the deep-living individual. Note how changes in filter pigments greatly affect tuning of underlying photoreceptors. The spectra were computed as for figure 3_b_.
Figure 7.
Photographs taken at various times of a cryosection from a retina of Neogonodactylus oerstedii in mounting medium on a microscope slide. The section shows a series of proximal filters from the third ommatidial row. (a) A photograph of the freshly mounted section. (b) The same section a few days later, and (c) again after several weeks. As the filter slowly denatured on the slide, its transmitted colour changed from blue through purple to red, following the same series of changes as the heated filter in figure 5_c_.
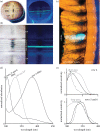
Figure 8.
Ultraviolet filters in Odontodactylus scyllarus. (a,b) The eye (a) and a magnified view of the midband region (b) of O. scyllarus under white (left panel) and ultraviolet (right panel) illumination. The fourth midband row fluoresces blue-green under ultraviolet illumination and overlays the dark pseudo-pupil, indicating that the fluorophore lies in the optical path. (c) A cross section of the midband of O. scyllarus with yellow transmitted illumination and ultraviolet epi-illumination, indicating that the blue-green fluorescence in midband row four is localized to the crystalline cones. (d) Normalized absorbance spectra of the crystalline cones in midband rows four, five and six (black lines), and the absorbance spectra of a hypothetical 330 nm visual pigment (VP) [12] expressed in the underlying R8s (grey line). The absorbance spectra were measured using microspectrophotometry with 200-μm-thick sections of the crystalline cone layer oriented along their optical path. (e) Tuning effects of the crystalline cone ultraviolet filters found in midband rows four (top panel) and five and six (bottom panel) on a hypothetical shared visual pigment with a _λ_max of 330 nm (grey line, plotting absorptance of the R8, computed using rhabdom lengths measured in [7]). The plot shows the modelled normalized sensitivity of the R8 (black line, computed from the absorptance of the R8 and the transmittance of the filter) versus the spectral sensitivities measured electrophysiologically (dashed line, adapted from [34]).
Similar articles
- Colour vision in stomatopod crustaceans.
Cronin TW, Porter ML, Bok MJ, Caldwell RL, Marshall J. Cronin TW, et al. Philos Trans R Soc Lond B Biol Sci. 2022 Oct 24;377(1862):20210278. doi: 10.1098/rstb.2021.0278. Epub 2022 Sep 5. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36058241 Free PMC article. Review. - Biological sunscreens tune polychromatic ultraviolet vision in mantis shrimp.
Bok MJ, Porter ML, Place AR, Cronin TW. Bok MJ, et al. Curr Biol. 2014 Jul 21;24(14):1636-1642. doi: 10.1016/j.cub.2014.05.071. Epub 2014 Jul 3. Curr Biol. 2014. PMID: 24998530 - Ultraviolet filters in stomatopod crustaceans: diversity, ecology and evolution.
Bok MJ, Porter ML, Cronin TW. Bok MJ, et al. J Exp Biol. 2015 Jul;218(Pt 13):2055-66. doi: 10.1242/jeb.122036. Epub 2015 May 11. J Exp Biol. 2015. PMID: 25964422 - The molecular genetics and evolution of colour and polarization vision in stomatopod crustaceans.
Cronin TW, Porter ML, Bok MJ, Wolf JB, Robinson PR. Cronin TW, et al. Ophthalmic Physiol Opt. 2010 Sep;30(5):460-9. doi: 10.1111/j.1475-1313.2010.00762.x. Ophthalmic Physiol Opt. 2010. PMID: 20883329 Review. - Evolution of anatomical and physiological specialization in the compound eyes of stomatopod crustaceans.
Porter ML, Zhang Y, Desai S, Caldwell RL, Cronin TW. Porter ML, et al. J Exp Biol. 2010 Oct 15;213(Pt 20):3473-86. doi: 10.1242/jeb.046508. J Exp Biol. 2010. PMID: 20889828
Cited by
- Optic lobe organization in stomatopod crustacean species possessing different degrees of retinal complexity.
Lin C, Chou A, Cronin TW. Lin C, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Mar;206(2):247-258. doi: 10.1007/s00359-019-01387-5. Epub 2019 Dec 6. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 31811397 - Exceptional diversity of opsin expression patterns in Neogonodactylus oerstedii (Stomatopoda) retinas.
Porter ML, Awata H, Bok MJ, Cronin TW. Porter ML, et al. Proc Natl Acad Sci U S A. 2020 Apr 21;117(16):8948-8957. doi: 10.1073/pnas.1917303117. Epub 2020 Apr 2. Proc Natl Acad Sci U S A. 2020. PMID: 32241889 Free PMC article. - Seeing and doing: how vision shapes animal behaviour.
Cronin TW, Douglas RH. Cronin TW, et al. Philos Trans R Soc Lond B Biol Sci. 2014 Jan 6;369(1636):20130030. doi: 10.1098/rstb.2013.0030. Print 2014. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24395959 Free PMC article. No abstract available. - Evolutionary history limits species' ability to match colour sensitivity to available habitat light.
Murphy MJ, Westerman EL. Murphy MJ, et al. Proc Biol Sci. 2022 May 25;289(1975):20220612. doi: 10.1098/rspb.2022.0612. Epub 2022 May 18. Proc Biol Sci. 2022. PMID: 35582803 Free PMC article. - Colour vision in stomatopod crustaceans.
Cronin TW, Porter ML, Bok MJ, Caldwell RL, Marshall J. Cronin TW, et al. Philos Trans R Soc Lond B Biol Sci. 2022 Oct 24;377(1862):20210278. doi: 10.1098/rstb.2021.0278. Epub 2022 Sep 5. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36058241 Free PMC article. Review.
References
- Caldwell RL, Dingle H. 1975. Ecology and evolution of agonistic behavior in stomatopods. Naturwissenschaften 65, 214–222. (10.1007/BF00603166) - DOI
- Manning RB, Schiff H, Abbott BC. 1984. Eye structure and the classification of stomatopod Crustacea. Zool. Scr. 13, 41–44. (10.1111/j.1463-6409.1984.tb00021.x) - DOI
- Harling C. 2000. Re-examination of eye design in the classification of stomatopod crustaceans. J. Crust. Biol. 20, 172–185.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical