Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice - PubMed (original) (raw)
. 2014 Apr;146(4):1108-18.
doi: 10.1053/j.gastro.2013.12.035. Epub 2014 Jan 5.
Xinbao Hao 2, Jun Qin 3, Wenhua Tang 4, Fengtian He 5, Amber Smith 6, Min Zhang 5, Diane M Simeone 7, Xiaotan T Qiao 8, Zhi-Nan Chen 9, Theodore S Lawrence 5, Liang Xu 10
Affiliations
- PMID: 24397969
- PMCID: PMC3982149
- DOI: 10.1053/j.gastro.2013.12.035
Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice
Ling Li et al. Gastroenterology. 2014 Apr.
Abstract
Background & aims: CD44s is a surface marker of tumor-initiating cells (TICs); high tumor levels correlate with metastasis and recurrence, as well as poor outcomes for patients. Monoclonal antibodies against CD44s might eliminate TICs with minimal toxicity. This strategy is unclear for treatment of pancreatic cancer, and little is known about how anti-CD44s affect pancreatic cancer initiation or recurrence after radiotherapy.
Methods: One hundred ninety-two pairs of human pancreatic adenocarcinoma and adjacent nontumor pancreatic tissues were collected from patients undergoing surgery. We measured CD44s levels in tissue samples and pancreatic cancer cell lines by immunohistochemistry, real-time polymerase chain reaction, and immunoblot; levels were correlated with patient survival times. We studied the effects of anti-CD44s in mice with human pancreatic tumor xenografts and used flow cytometry to determine the effects on TICs. Changes in CD44s signaling were examined by real-time polymerase chain reaction, immunoblot, reporter assay, and in vitro tumorsphere formation assays.
Results: Levels of CD44s were significantly higher in pancreatic cancer than adjacent nontumor tissues. Patients whose tumors expressed high levels of CD44s had a median survival of 10 months compared with >43 months for those with low levels. Anti-CD44s reduced growth, metastasis, and postradiation recurrence of pancreatic xenograft tumors in mice. The antibody reduced the number of TICs in cultured pancreatic cancer cells and xenograft tumors, as well as their tumorigenicity. In cultured pancreatic cancer cell lines, anti-CD44s down-regulated the stem cell self-renewal genes Nanog, Sox-2, and Rex-1 and inhibited signal transducer and activator of transcription 3-mediated cell proliferation and survival signaling.
Conclusions: The TIC marker CD44s is up-regulated in human pancreatic tumors and associated with patient survival time. CD44s is required for initiation, growth, metastasis, and postradiation recurrence of xenograft tumors in mice. Anti-CD44s eliminated bulk tumor cells as well as TICs from the tumors. Strategies to target CD44s cab be developed to block pancreatic tumor formation and post-radiotherapy recurrence in patients.
Keywords: Cancer Stem Cell; H4C4; Progression; Tumorigenesis.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Author disclosures: The authors declare no conflicts of interest.
Figures
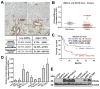
Figure 1. CD44s is upregulated in human pancreatic cancer and is correlated with patients’ overall survival
(A) Representative CD44s immunohistochemistry staining in pancreatic cancer (lower panel) and adjacent non-tumor tissues (top panel). Bar, 50 μm. Magnification: 400X. Estimated by χ2 test, P < 0.001, compared to normal pancreas. (B) Relative CD44s mRNA levels in 36 pairs of pancreatic cancer and adjacent non-tumor tissues. Data was normalized to 18sRNA/GAPDH. (C) Kaplan-Meier survival analysis of overall survival in 66 patients comparing high and low CD44s expression groups. (D) CD44s mRNA expression in human pancreatic cancer cell lines, tested using qRT-PCR. (E) Western blot of CD44s protein in human pancreatic cancer cell lines. β-actin: loading control.
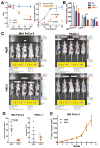
Figure 2. H4C4 inhibits pancreatic tumor growth in vitro and in vivo
(A) In vitro cell growth assay. Cells were treated with H4C4 and nIgG at different doses (0–10μg/mL) and time points (24–96 hours). Cell viability was determined by WST-8 assay (left) and cell counting (right). (B) In vitro cell invasion assay. Cells were treated by 10 μg/mL H4C4 or nIgG. NT, no treatment. (C–D) In vivo tumor growth assay. MIA PaCa-2-luc (left) and PANC-1-luc (right) cells were injected into the tail of pancreas of nude mice (2×106 cells/site, n = 5), 4 mg/kg H4C4 or nIgG were injected i.v. the following day, three times per week for 8 weeks. (C) Bioluminescence imaging analysis of the xenograft mice. Quantifiable photon emission from in vivo luciferase signaling was recorded using IVIS system. Note the color scale bars are different in scale, as marked in yellow box. (D) Change of tumor growth as monitored by BLI luciferase activity at 4 weeks. (E) In vivo tumor formation and tumor growth in mice injected with Mia PaCa-2 cells pre-treated with 8 μg/mL of H4C4 or nIgG (1×106 cells/site, n=10).
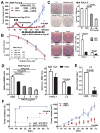
Figure 3. H4C4 inhibits post-radiation tumor recurrence by inhibiting pancreatic TICs
(A) H4C4-mediated inhibition of post-radiation tumor recurrence. MIA PaCa-2 s.q. xenografts at 150 mm3 received daily 2 Gy X-ray radiation (while shielding the rest of the body), total 20 Gy. On day 49, the mice with no palpable tumors were randomized into two groups and started treatment with either H4C4 or nIgG, 4 mg/kg i.v., twice a week. (B) Clonogenic survival assay of MIA PaCa-2 cells treated by 10 and 20 μg/ml H4C4 or nIgG. Survival fractions were plotted and the enhancement ratios (ER) by H4C4 were calculated vs. nIgG. (C) Colony formation assay of MIA PaCa-2 cells treated 5 and 10 μg/ml H4C4 or 10 μg/ml nIgG. Representative pictures of clones were shown. (D) Tumorphere formation assay of 1, 2, 5, 10 μg/ml H4C4 or 10 μg/ml nIgG treated cells. (E) Flow cytometry analysis of CD44+CD24+ESA+ subpopulation in human primary pancreatic cancer early passage J2 xenografts treated with H4C4 or nIgG. (F) Tumor formation and tumor growth evaluation in NOD-SCID mice injected with the sorted CD44+CD24+ESA+ subpopulation of J-2 xenografts treated with 4 mg/kg of H4C4 or nIgG.
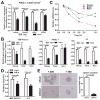
Figure 4. H4C4 inhibits TICs by regulating Nanog signaling
(A) Nanog, Smad2, GJA1 and STAT3 mRNA expression in the CD44+CD133+ PANC-1 cells after H4C4 or nIgG treatment. Data were normalized to GAPDH. (B) Nanog, Sox2 and Rex1 mRNA expression in MIA PaCa-2, PANC-1 and J-2 cells after H4C4 or nIgG treatment. Data was normalized to β-actin. (C) Time-course of Nanog, Sox2, and Rex1 mRNA expression in J-2 cells after H4C4 treatment. (D) Sox2 transcriptional activity in MIA PaCa-2 and J-2 cells. Cells were treated by 10 μg/mL H4C4 or nIgG. Dual Luciferase assay was performed, a Renilla reporter was used for internal normalization. (E) Tumorphere formation assay in Sox2 knock-down cells.
Figure 5. H4C4 inhibits bulk tumor cells by regulating STAT3 signaling
(A) Apoptosis analysis in MIA PaCa-2 cells treated with H4C4 or nIgG using Annexin V-FITC/PI staining. (B) STAT3, cyclin D1, survivin and XIAP mRNA expression in MIA PaCa-2, PANC-1 and J-2 cells after H4C4 or nIgG treatment. Data were normalized to GAPDH. (C) Protein levels of pSTAT3, total STAT3, cyclin D1, survivin and XIAP in MIA PaCa-2, PANC-1 and J-2 cells after H4C4 or nIgG treatment. Cells were collected at 0, 15, 30, 60, 120, and 240 minutes post-treatment. (D) STAT3 transcriptional activity in MIA PaCa-2 and PANC-1 cells after H4C4 or nIgG treatment. The luciferase activity was measured using the Bright-Glo™ Luciferase Assay System, with β-galactosidase as internal control. (E): Tumorsphere formation assay in MIA PaCa-2 cells after the treatment with STAT3 inhibitor FLLL32 (5 μM) or survivin inhibitor YM155 (0.1 μM) alone or in combination with H4C4 (10 μg/ml).
Figure 6. A proposed working model for H4C4
H4C4 deactivates bulk tumor cell survival partly by inhibiting STAT3 signalling pathway, and reduces TICs and its capacity for tumor initiation by downregulating stem cell self-renewal genes Nanog/Sox2/Rex1.
Comment in
- Pancreatic cancer: pancreatic tumour formation and recurrence after radiotherapy are blocked by targeting CD44.
Wood NJ. Wood NJ. Nat Rev Gastroenterol Hepatol. 2014 Feb;11(2):73. doi: 10.1038/nrgastro.2014.1. Epub 2014 Jan 21. Nat Rev Gastroenterol Hepatol. 2014. PMID: 24445614 Review. No abstract available.
Similar articles
- JTC801 Induces pH-dependent Death Specifically in Cancer Cells and Slows Growth of Tumors in Mice.
Song X, Zhu S, Xie Y, Liu J, Sun L, Zeng D, Wang P, Ma X, Kroemer G, Bartlett DL, Billiar TR, Lotze MT, Zeh HJ, Kang R, Tang D. Song X, et al. Gastroenterology. 2018 Apr;154(5):1480-1493. doi: 10.1053/j.gastro.2017.12.004. Epub 2017 Dec 14. Gastroenterology. 2018. PMID: 29248440 Free PMC article. - Inhibition of Tumor Growth and Metastasis in Pancreatic Cancer Models by Interference With CD44v6 Signaling.
Matzke-Ogi A, Jannasch K, Shatirishvili M, Fuchs B, Chiblak S, Morton J, Tawk B, Lindner T, Sansom O, Alves F, Warth A, Schwager C, Mier W, Kleeff J, Ponta H, Abdollahi A, Orian-Rousseau V. Matzke-Ogi A, et al. Gastroenterology. 2016 Feb;150(2):513-25.e10. doi: 10.1053/j.gastro.2015.10.020. Epub 2015 Oct 24. Gastroenterology. 2016. PMID: 26597578 - HAb18G/CD147 promotes pSTAT3-mediated pancreatic cancer development via CD44s.
Li L, Tang W, Wu X, Karnak D, Meng X, Thompson R, Hao X, Li Y, Qiao XT, Lin J, Fuchs J, Simeone DM, Chen ZN, Lawrence TS, Xu L. Li L, et al. Clin Cancer Res. 2013 Dec 15;19(24):6703-15. doi: 10.1158/1078-0432.CCR-13-0621. Epub 2013 Oct 16. Clin Cancer Res. 2013. PMID: 24132924 Free PMC article. - Pancreatic cancer: pancreatic tumour formation and recurrence after radiotherapy are blocked by targeting CD44.
Wood NJ. Wood NJ. Nat Rev Gastroenterol Hepatol. 2014 Feb;11(2):73. doi: 10.1038/nrgastro.2014.1. Epub 2014 Jan 21. Nat Rev Gastroenterol Hepatol. 2014. PMID: 24445614 Review. No abstract available. - Pancreatic cancer stem cells.
Lee CJ, Dosch J, Simeone DM. Lee CJ, et al. J Clin Oncol. 2008 Jun 10;26(17):2806-12. doi: 10.1200/JCO.2008.16.6702. J Clin Oncol. 2008. PMID: 18539958 Review.
Cited by
- Proteoglycan serglycin promotes non-small cell lung cancer cell migration through the interaction of its glycosaminoglycans with CD44.
Guo JY, Chiu CH, Wang MJ, Li FA, Chen JY. Guo JY, et al. J Biomed Sci. 2020 Jan 2;27(1):2. doi: 10.1186/s12929-019-0600-3. J Biomed Sci. 2020. PMID: 31898491 Free PMC article. - Niclosamide improves cancer immunotherapy by modulating RNA-binding protein HuR-mediated PD-L1 signaling.
Zhang Q, Yang Z, Hao X, Dandreo LJ, He L, Zhang Y, Wang F, Wu X, Xu L. Zhang Q, et al. Cell Biosci. 2023 Oct 17;13(1):192. doi: 10.1186/s13578-023-01137-w. Cell Biosci. 2023. PMID: 37848943 Free PMC article. - The Role of lncRNAs in the Stem Phenotype of Pancreatic Ductal Adenocarcinoma.
Melendez-Zajgla J, Maldonado V. Melendez-Zajgla J, et al. Int J Mol Sci. 2021 Jun 15;22(12):6374. doi: 10.3390/ijms22126374. Int J Mol Sci. 2021. PMID: 34203589 Free PMC article. Review. - CD44: A New Prognostic Marker in Colorectal Cancer?
Ziranu P, Pretta A, Aimola V, Cau F, Mariani S, D'Agata AP, Codipietro C, Rizzo D, Dell'Utri V, Sanna G, Moledda G, Cadoni A, Lai E, Puzzoni M, Pusceddu V, Castagnola M, Scartozzi M, Faa G. Ziranu P, et al. Cancers (Basel). 2024 Apr 19;16(8):1569. doi: 10.3390/cancers16081569. Cancers (Basel). 2024. PMID: 38672650 Free PMC article. Review. - Altering the response to radiation: radiosensitizers and targeted therapies in pancreatic ductal adenocarcinoma: preclinical and emerging clinical evidence.
Wolfe AR, Williams TM. Wolfe AR, et al. Ann Pancreat Cancer. 2018 Aug;1(8):26. doi: 10.21037/apc.2018.08.02. Epub 2018 Aug 31. Ann Pancreat Cancer. 2018. PMID: 32656528 Free PMC article.
References
- Gutt R, Liauw SL, Weichselbaum RR. The role of radiotherapy in locally advanced pancreatic carcinoma. Nat Rev Gastroenterol Hepatol. 2010;7:437–47. - PubMed
- Trakul N, Koong AC, Maxim PG, et al. Modern radiation therapy techniques for pancreatic cancer. Gastroenterol Clin North Am. 2012;41:223–35. - PubMed
- Zller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nature Reviews Cancer. 2011;11:254–267. - PubMed
Supplementary References
- Chang C, Takayanagi A, Yoshida T, et al. Screening of scFv-displaying phages recognizing distinct extracellular domains of EGF receptor by target-guided proximity labeling method. J Immunol Methods. 2011;372:127–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA121830 S1/CA/NCI NIH HHS/United States
- R01 CA121830/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- P20 GM103418/GM/NIGMS NIH HHS/United States
- 5 P30 CA46592/CA/NCI NIH HHS/United States
- R01 CA134655/CA/NCI NIH HHS/United States
- CA134655/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous