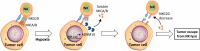The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity - PubMed (original) (raw)
Review
The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity
Joanna Baginska et al. Front Immunol. 2013.
Abstract
Considerable evidence has been gathered over the last 10 years showing that the tumor microenvironment (TME) is not simply a passive recipient of immune cells, but an active participant in the establishment of immunosuppressive conditions. It is now well documented that hypoxia, within the TME, affects the functions of immune effectors including natural killer (NK) cells by multiple overlapping mechanisms. Indeed, each cell in the TME, irrespective of its transformation status, has the capacity to adapt to the hostile TME and produce immune modulatory signals or mediators affecting the function of immune cells either directly or through the stimulation of other cells present in the tumor site. This observation has led to intense research efforts focused mainly on tumor-derived factors. Notably, it has become increasingly clear that tumor cells secrete a number of environmental factors such as cytokines, growth factors, exosomes, and microRNAs impacting the immune cell response. Moreover, tumor cells in hostile microenvironments may activate their own intrinsic resistance mechanisms, such as autophagy, to escape the effective immune response. Such adaptive mechanisms may also include the ability of tumor cells to modify their metabolism and release several metabolites to impair the function of immune cells. In this review, we summarize the different mechanisms involved in the TME that affect the anti-tumor immune function of NK cells.
Keywords: autophagy; hypoxia; natural killer cells; tumor microenvironment; tumor-derived exosomes.
Figures
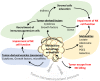
Figure 1
The tumor microenvironment activates different mechanisms to impair the NK-mediated anti-tumor immunity. Under the pressure of the tumor microenvironment (TME), tumor cells adapt to such stress by activating intrinsic resistance mechanisms (autophagy) or by regulating their metabolism. Such regulation leads to the secretion of several metabolites that impair the function of NK cells in the tumor site (yellow area). Tumor cells under stress conditions may activate the release of tumor-derived vesicles containing cytokines, growth factors, or microRNAs to directly impact the NK functions (blue area). Such factors can be secreted directly in the TME to recruit immunosuppressive cells or to educate stromal cells involved in the impairment of NK cell functions (green area).
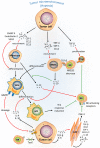
Figure 2
Complex cellular interplay within the hypoxic tumor microenvironment inhibits NK-mediated killing. Tumor cells in a hypoxic tumor microenvironment (TME) secrete soluble factors that educate immune cells [e.g., monocytes, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Treg)], and stromal cells such as cancer-associated fibroblasts (CAFs). This scheme summarizes the effects of tumor-derived soluble factors on recruitment, differentiation, proliferation, and activation of tumor-associated cells (red arrows) in the hypoxic TME and their immunosuppressive activities (green lines) on NK-mediated lysis of tumor cells.
Figure 3
Soluble MICA/B regulate NKG2D receptors on the surface of NK cells. Under hypoxic stress, tumor cells activate expression through HIF-1α and the release of ADAM10. Released ADAM10 cleaves MICA/B ligands on the surface of tumor cells and soluble MICA/B downregulates the expression of NKG2D on the surface of NK cells, leading to tumor escape from NK-mediated killing.
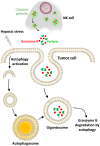
Figure 4
Hypoxic stress activates autophagy in tumor cells as an intrinsic resistance mechanism to NK-mediated killing. In our model, the cytolytic effectors perforin and granzyme B enter the target cells by endocytosis and then are found in enlarged endosomes called “gigantosomes.” In hypoxic cells, the activation of autophagy leads to the formation of autophagosomes that fuse with “gigantosomes” to form amphisomes. The fusion between amphisomes and lysosomes selectively degrades granzyme B in this compartment, making hypoxic tumor cells less sensitive to NK-mediated lysis.
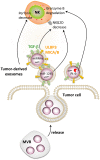
Figure 5
Impairment of NK cell function by tumor-derived exosomes. Tumor cells secrete extracellular vesicles called exosomes. Tumor-derived exosomes contain numerous factors able to modulate the function of NK cells such as MICA/B, ULBP3, TGF-β, PI-9, and different microRNAs. Exosome-derived MICA/B, ULBP3, TGF-β, and miR-1245 can decrease NKG2D on the surface of NK cells, while PI-9 degrades granzyme B. Tumor-derived exosomes can also decrease the level of perforin in NK cells by a still-unknown mechanism.
Similar articles
- Sculpting tumor microenvironment with immune system: from immunometabolism to immunoediting.
Yu YR, Ho PC. Yu YR, et al. Clin Exp Immunol. 2019 Aug;197(2):153-160. doi: 10.1111/cei.13293. Epub 2019 Apr 1. Clin Exp Immunol. 2019. PMID: 30873592 Free PMC article. Review. - Cytokines Orchestrating the Natural Killer-Myeloid Cell Crosstalk in the Tumor Microenvironment: Implications for Natural Killer Cell-Based Cancer Immunotherapy.
Gaggero S, Witt K, Carlsten M, Mitra S. Gaggero S, et al. Front Immunol. 2021 Jan 29;11:621225. doi: 10.3389/fimmu.2020.621225. eCollection 2020. Front Immunol. 2021. PMID: 33584718 Free PMC article. Review. - Intrinsic and extrinsic factors determining natural killer cell fate: Phenotype and function.
Zhi L, Wang X, Gao Q, He W, Shang C, Guo C, Niu Z, Zhu W, Zhang X. Zhi L, et al. Biomed Pharmacother. 2023 Sep;165:115136. doi: 10.1016/j.biopha.2023.115136. Epub 2023 Jul 13. Biomed Pharmacother. 2023. PMID: 37453199 Review. - Critical Role of Tumor Microenvironment in Shaping NK Cell Functions: Implication of Hypoxic Stress.
Hasmim M, Messai Y, Ziani L, Thiery J, Bouhris JH, Noman MZ, Chouaib S. Hasmim M, et al. Front Immunol. 2015 Sep 23;6:482. doi: 10.3389/fimmu.2015.00482. eCollection 2015. Front Immunol. 2015. PMID: 26441986 Free PMC article. Review. - Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy?
Ben-Shmuel A, Biber G, Barda-Saad M. Ben-Shmuel A, et al. Front Immunol. 2020 Feb 21;11:275. doi: 10.3389/fimmu.2020.00275. eCollection 2020. Front Immunol. 2020. PMID: 32153582 Free PMC article. Review.
Cited by
- Simultaneous editing of TCR, HLA-I/II and HLA-E resulted in enhanced universal CAR-T resistance to allo-rejection.
Li W, Zhu X, Xu Y, Chen J, Zhang H, Yang Z, Qi Y, Hong J, Li Y, Wang G, Shen J, Qian C. Li W, et al. Front Immunol. 2022 Dec 2;13:1052717. doi: 10.3389/fimmu.2022.1052717. eCollection 2022. Front Immunol. 2022. PMID: 36532006 Free PMC article. - Molecular mechanisms regulating cytotoxic lymphocyte development and function, and their associations to human diseases.
Krzewski K, Bryceson YT. Krzewski K, et al. Front Immunol. 2014 Jun 11;5:279. doi: 10.3389/fimmu.2014.00279. eCollection 2014. Front Immunol. 2014. PMID: 24966858 Free PMC article. No abstract available. - The multifaceted role of autophagy in tumor evasion from immune surveillance.
Janji B, Viry E, Moussay E, Paggetti J, Arakelian T, Mgrditchian T, Messai Y, Noman MZ, Van Moer K, Hasmim M, Mami-Chouaib F, Berchem G, Chouaib S. Janji B, et al. Oncotarget. 2016 Apr 5;7(14):17591-607. doi: 10.18632/oncotarget.7540. Oncotarget. 2016. PMID: 26910842 Free PMC article. Review. - Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma.
Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC. Zhu L, et al. Theranostics. 2017 Jul 7;7(10):2732-2745. doi: 10.7150/thno.18752. eCollection 2017. Theranostics. 2017. PMID: 28819459 Free PMC article. - Optimising NK cell metabolism to increase the efficacy of cancer immunotherapy.
Choi C, Finlay DK. Choi C, et al. Stem Cell Res Ther. 2021 Jun 5;12(1):320. doi: 10.1186/s13287-021-02377-8. Stem Cell Res Ther. 2021. PMID: 34090499 Free PMC article. Review.
References
- Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol (2004) 173(6):3716–24 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources