Increasing value and reducing waste: addressing inaccessible research - PubMed (original) (raw)
Increasing value and reducing waste: addressing inaccessible research
An-Wen Chan et al. Lancet. 2014.
Abstract
The methods and results of health research are documented in study protocols, full study reports (detailing all analyses), journal reports, and participant-level datasets. However, protocols, full study reports, and participant-level datasets are rarely available, and journal reports are available for only half of all studies and are plagued by selective reporting of methods and results. Furthermore, information provided in study protocols and reports varies in quality and is often incomplete. When full information about studies is inaccessible, billions of dollars in investment are wasted, bias is introduced, and research and care of patients are detrimentally affected. To help to improve this situation at a systemic level, three main actions are warranted. First, academic institutions and funders should reward investigators who fully disseminate their research protocols, reports, and participant-level datasets. Second, standards for the content of protocols and full study reports and for data sharing practices should be rigorously developed and adopted for all types of health research. Finally, journals, funders, sponsors, research ethics committees, regulators, and legislators should endorse and enforce policies supporting study registration and wide availability of journal reports, full study reports, and participant-level datasets.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
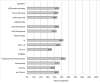
Figure 1
Publication rates for positive studies versus null/negative studies, by type of study cohort (12 inception cohorts of 2,531 protocols; 4 cohorts of 855 regulatory agency submissions; 27 cohorts of 10,289 conference abstracts; and 4 cohorts of 2,636 manuscripts submitted to journals).,
Figure 2
Publication rates for random sample of 677 completed trials registered on
ClinicalTrials.gov
from 2000-2007, by study characteristic. Adapted from Ross J et al.
Figure 3
Results of published versus unpublished randomised trials of reboxetine versus placebo for acute treatment of major depression. Adapted from Eyding D et al.
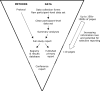
Figure 4
Key sources of information on study methods and results, with associated information loss and potential for selective reporting.
Comment in
- Research: increasing value, reducing waste.
Völzke H, Schmidt CO, Hoffmann W. Völzke H, et al. Lancet. 2014 Mar 29;383(9923):1124. doi: 10.1016/S0140-6736(14)60559-6. Lancet. 2014. PMID: 24679622 No abstract available. - Research: increasing value, reducing waste - Authors' reply.
Glasziou P, Macleod M, Chalmers I, Ioannidis JP, Al-Shahi Salman R, Chan AW. Glasziou P, et al. Lancet. 2014 Mar 29;383(9923):1126-7. doi: 10.1016/S0140-6736(14)60563-8. Lancet. 2014. PMID: 24679627 No abstract available. - Review and publication of protocol submissions to Trials - what have we learned in 10 years?
Li T, Boutron I, Al-Shahi Salman R, Cobo E, Flemyng E, Grimshaw JM, Altman DG. Li T, et al. Trials. 2016 Dec 16;18(1):34. doi: 10.1186/s13063-016-1743-0. Trials. 2016. PMID: 28114958 Free PMC article.
Similar articles
- Reducing waste from incomplete or unusable reports of biomedical research.
Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, Michie S, Moher D, Wager E. Glasziou P, et al. Lancet. 2014 Jan 18;383(9913):267-76. doi: 10.1016/S0140-6736(13)62228-X. Epub 2014 Jan 8. Lancet. 2014. PMID: 24411647 - A systematic review of comparisons between protocols or registrations and full reports in primary biomedical research.
Li G, Abbade LPF, Nwosu I, Jin Y, Leenus A, Maaz M, Wang M, Bhatt M, Zielinski L, Sanger N, Bantoto B, Luo C, Shams I, Shahid H, Chang Y, Sun G, Mbuagbaw L, Samaan Z, Levine MAH, Adachi JD, Thabane L. Li G, et al. BMC Med Res Methodol. 2018 Jan 11;18(1):9. doi: 10.1186/s12874-017-0465-7. BMC Med Res Methodol. 2018. PMID: 29325533 Free PMC article. - Noncommercial US Funders' Policies on Trial Registration, Access to Summary Results, and Individual Patient Data Availability.
Whitlock EP, Dunham KM, DiGioia K, Lazowick E, Gleason TC, Atkins D. Whitlock EP, et al. JAMA Netw Open. 2019 Jan 4;2(1):e187498. doi: 10.1001/jamanetworkopen.2018.7498. JAMA Netw Open. 2019. PMID: 30681715 Free PMC article. - [Transparency in clinical research: registration of clinical trials and publication of results].
Pérez-Mañá C, Llonch C, Farré M. Pérez-Mañá C, et al. Med Clin (Barc). 2012 Dec 1;139(13):593-7. doi: 10.1016/j.medcli.2012.06.009. Epub 2012 Sep 14. Med Clin (Barc). 2012. PMID: 22982127 Review. Spanish. No abstract available. - European Medicines Agency Policy 0070: an exploratory review of data utility in clinical study reports for academic research.
Ferran JM, Nevitt SJ. Ferran JM, et al. BMC Med Res Methodol. 2019 Nov 5;19(1):204. doi: 10.1186/s12874-019-0836-3. BMC Med Res Methodol. 2019. PMID: 31690260 Free PMC article. Review.
Cited by
- How do we create, and improve, the evidence base?
Innes NP, Schwendicke F, Lamont T. Innes NP, et al. Br Dent J. 2016 Jun 24;220(12):651-5. doi: 10.1038/sj.bdj.2016.451. Br Dent J. 2016. PMID: 27338909 - Research Waste.
Sogi GM. Sogi GM. Contemp Clin Dent. 2023 Jul-Sep;14(3):179. doi: 10.4103/ccd.ccd_434_23. Epub 2023 Oct 24. Contemp Clin Dent. 2023. PMID: 38075541 Free PMC article. No abstract available. - Impact of investigator initiated trials and industry sponsored trials on medical practice (IMPACT): rationale and study design.
Nury E, Bischoff K, Wollmann K, Nitschke K, Lohner S, Schumacher M, Rücker G, Blümle A. Nury E, et al. BMC Med Res Methodol. 2020 Oct 2;20(1):246. doi: 10.1186/s12874-020-01125-5. BMC Med Res Methodol. 2020. PMID: 33008297 Free PMC article. - Harmonization-Information Trade-Offs for Sharing Individual Participant Data in Biomedicine.
Torres-Espín A, Ferguson AR. Torres-Espín A, et al. Harv Data Sci Rev. 2022 Summer;4(3):10.1162/99608f92.a9717b34. doi: 10.1162/99608f92.a9717b34. Epub 2022 Jul 28. Harv Data Sci Rev. 2022. PMID: 36420049 Free PMC article. - A tutorial on methodological studies: the what, when, how and why.
Mbuagbaw L, Lawson DO, Puljak L, Allison DB, Thabane L. Mbuagbaw L, et al. BMC Med Res Methodol. 2020 Sep 7;20(1):226. doi: 10.1186/s12874-020-01107-7. BMC Med Res Methodol. 2020. PMID: 32894052 Free PMC article. Review.
References
- Chalmers I. Underreporting research is scientific misconduct. JAMA. 1990;263:1405–8. - PubMed
- Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14:iii–193. - PubMed
- Røttingen JA, Regmi S, Eide M, Young AJ, Viergever RF, Ardal C, et al. Mapping of available health research and development data: what's there, what's missing, and what role is there for a global observatory? Lancet. 2013;382:1286–307. - PubMed
- Galsworthy MJ, Hristovski D, Lusa L, Ernst K, Irwin R, Charlesworth K, et al. The academic output of nine years of EU investment into health research. Lancet. 2012;380:971–2. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources