Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model - PubMed (original) (raw)
Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model
Timothy W Lefever et al. Pharmacol Biochem Behav. 2014 Mar.
Abstract
Because Δ(9)-tetrahydrocannabinol (THC) has been a false negative in rat intravenous self-administration procedures, the evaluation of the abuse potential of candidate cannabinoid medications has proved difficult. One lab group has successfully trained self-administration of the aminoalkylindole WIN55,212-2 in rats; however, their results have not been independently replicated. The purpose of this study was to extend their model by using a within-subjects design, with the goal of establishing a robust method suitable for substitution testing of other cannabinoids. Male Long-Evans rats were trained to self-administer WIN55,212-2 (0.01 mg/kg/infusion) on a fixed ratio 3 schedule. Dose-effect curves for WIN55,212-2 were determined, followed by vehicle substitution and a dose-effect curve with THC. WIN55,212-2 self-administration was acquired; however, substitution with THC did not maintain responding above vehicle levels. Dose-dependent attenuation by rimonabant confirmed CB1 receptor mediation of WIN55,212-2's reinforcing effects. Vehicle substitution resulted in a session-dependent decrease in responding (i.e., extinction). While this study provides systematic replication of previous studies, lack of substitution with THC is problematic and suggests that WIN55,212-2 self-administration may be of limited usefulness as a screening tool for detection of the reinforcing effects of potential cannabinoid medications. Clarification of underlying factors responsible for failure of THC to maintain self-administration in cannabinoid-trained rats is needed.
Keywords: Abuse liability; Cannabinoids; Rats; Self-administration; WIN55,212-2; Δ(9)-Tetrahydrocannabinol.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest: There are no conflicts of interest.
Figures
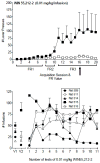
Figure 1
Top panel
: Acquisition of i.v. self-administration of 0.01 mg/kg/infusion WIN55,212-2 in adult male Long Evans rats. Number of presses on the active (filled symbols) and inactive (unfilled symbols) levers is shown as a function of number of acquisition sessions and value of the fixed ratio. Daily pre-session priming with 3 infusions of the training dose was initiated on session 16, as indicated by the arrow, and continued throughout the remainder of the experiment. Each point represents the mean (± SEM) number of lever presses for 5 rats.
Bottom panel
: Number of infusions of 0.01 mg/kg/infusion WIN55,212-2 for individual subjects during tests with the training dose or vehicle (left side of panel) throughout the study. Each point represents the mean number of infusions (± SEM) for a single rat during five sessions of WIN55,212-2 (0.01 mg/kg) or vehicle availability at 11 different times across the study. Points above V1 and V2 represent mean number of infusions (± SEM) of vehicle during separate exposures that occurred at two different time points during the study.
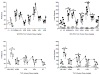
Figure 2
Substitution tests with various doses of WIN55,212-2 (top panels) and THC (bottom panels). For both drugs, each of 5 substitution tests with each dose (occurring on 5 consecutive sessions) is shown. The top panels show number of infusions (left panel) and active and inactive lever presses (right panel) as a function of WIN55,212-2 dose at the beginning (center section, circles) and end of the experiment (right section, squares). Points above V1 and V2 (left sections of each top panel) represent the results of control tests with vehicle conducted prior to the start of the first and second dose-effect curve determinations, respectively. The bottom panels show number of infusions of THC (left panel) and active and inactive lever presses (right panel) as a function of THC dose (right section). Points above Veh and WIN (left sections of each bottom panel) represent the results of control tests with vehicle and the training dose (0.01 mg/kg/infusion WIN55,212-2), respectively, conducted prior to the start of the dose-effect curve determination. In both right panels, filled symbols represent responses on the active lever and unfilled symbols represent responses on inactive lever. All values represent the mean (±SEM) of data from 5 rats. *P < 0.05 indicates significant dose X day (top left panel) or significant dose X lever (both right panels) interaction and post hoc difference compared to mean rates of infusions or active lever responding during the corresponding control test with vehicle. # P < 0.05 indicates significant dose X lever interaction and post hoc difference compared to presses on the inactive lever at the same dose. $ P < 0.05 indicates a significant main effect of dose.
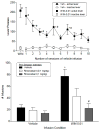
Figure 3
Top panel
: Effects of substitution of vehicle for the WIN55,212-2 training dose over a period of 10 consecutive sessions on lever presses on the active and inactive levers. At the left side of the panel, responding during test sessions with WIN55,212-2 (0.01 mg/kg/infusion) is shown for comparison purposes. All values represent the mean (±SEM) of data from 5 rats. ANOVA revealed a significant interaction. Post-hoc analysis of the simple effects: * indicates a significant difference (p < 0.05) in number of lever presses on the active lever on a vehicle session compared to active lever presses during WIN55,212-2 sessions. # designates a significant difference (p < 0.05) between number of presses on the active lever compared to the inactive lever during the same session.
Bottom panel
: Effects of pre-session administration of rimonabant (0.1 and 1 mg/kg) on responding on the active lever in rats trained to self-administer 0.01 mg/kg/infusion WIN55,212-2. The left side of the top panel shows results of pre-session injections on number of infusions when vehicle was available for infusion. The right side of the top panel shows results of the injections on number of infusions when 0.01 mg/kg WIN55,212-2 was available for infusion. Values represent the mean (±SEM) of data from 5 rats. ANOVA revealed a significant interaction. Post-hoc analysis of the simple effects: * designates a significant (p < 0.05) difference compared to the corresponding test with vehicle and # indicates a significant (p < 0.05) difference compared to the training dose of WIN55,212-2.
Similar articles
- Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats.
Fattore L, Cossu G, Martellotta CM, Fratta W. Fattore L, et al. Psychopharmacology (Berl). 2001 Aug;156(4):410-6. doi: 10.1007/s002130100734. Psychopharmacology (Berl). 2001. PMID: 11498718 - The hypocretin/orexin receptor-1 as a novel target to modulate cannabinoid reward.
Flores Á, Maldonado R, Berrendero F. Flores Á, et al. Biol Psychiatry. 2014 Mar 15;75(6):499-507. doi: 10.1016/j.biopsych.2013.06.012. Epub 2013 Jul 26. Biol Psychiatry. 2014. PMID: 23896204 - Cannabinoid self-administration in rats: sex differences and the influence of ovarian function.
Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Fattore L, et al. Br J Pharmacol. 2007 Nov;152(5):795-804. doi: 10.1038/sj.bjp.0707465. Epub 2007 Sep 24. Br J Pharmacol. 2007. PMID: 17891164 Free PMC article. - Self-administration of cannabinoids by experimental animals and human marijuana smokers.
Justinova Z, Goldberg SR, Heishman SJ, Tanda G. Justinova Z, et al. Pharmacol Biochem Behav. 2005 Jun;81(2):285-99. doi: 10.1016/j.pbb.2005.01.026. Pharmacol Biochem Behav. 2005. PMID: 15932767 Free PMC article. Review. - Study of cannabinoid dependence in animals.
Maldonado R. Maldonado R. Pharmacol Ther. 2002 Aug;95(2):153-64. doi: 10.1016/s0163-7258(02)00254-1. Pharmacol Ther. 2002. PMID: 12182962 Review.
Cited by
- Nicotine Enhances Intravenous Self-administration of Cannabinoids in Adult Rats.
Stringfield SJ, Sanders BE, Suppo JA, Sved AF, Torregrossa MM. Stringfield SJ, et al. Nicotine Tob Res. 2023 Apr 6;25(5):1022-1029. doi: 10.1093/ntr/ntac267. Nicotine Tob Res. 2023. PMID: 36426873 Free PMC article. - Consequences of Adolescent Exposure to the Cannabinoid Receptor Agonist WIN55,212-2 on Working Memory in Female Rats.
Kirschmann EK, McCalley DM, Edwards CM, Torregrossa MM. Kirschmann EK, et al. Front Behav Neurosci. 2017 Jul 21;11:137. doi: 10.3389/fnbeh.2017.00137. eCollection 2017. Front Behav Neurosci. 2017. PMID: 28785210 Free PMC article. - Dissecting the role of CB1 and CB2 receptors in cannabinoid reward versus aversion using transgenic CB1- and CB2-knockout mice.
Li X, Hempel BJ, Yang HJ, Han X, Bi GH, Gardner EL, Xi ZX. Li X, et al. Eur Neuropsychopharmacol. 2021 Feb;43:38-51. doi: 10.1016/j.euroneuro.2020.11.019. Epub 2020 Dec 15. Eur Neuropsychopharmacol. 2021. PMID: 33334652 Free PMC article. - In vitro and in vivo pharmacology of nine novel synthetic cannabinoid receptor agonists.
Marusich JA, Gamage TF, Zhang Y, Akinfiresoye LR, Wiley JL. Marusich JA, et al. Pharmacol Biochem Behav. 2022 Oct;220:173467. doi: 10.1016/j.pbb.2022.173467. Epub 2022 Sep 22. Pharmacol Biochem Behav. 2022. PMID: 36154844 Free PMC article. - Selective breeding for high alcohol preference is associated with increased sensitivity to cannabinoid reward within the nucleus accumbens shell.
Hauser SR, Katner SN, Waeiss RA, Truitt WA, Bell RL, McBride WJ, Rodd ZA. Hauser SR, et al. Pharmacol Biochem Behav. 2020 Oct;197:173002. doi: 10.1016/j.pbb.2020.173002. Epub 2020 Jul 23. Pharmacol Biochem Behav. 2020. PMID: 32710885 Free PMC article.
References
- Ator N, Griffiths R. Self-administration of barbiturates and benzodiazepines: A review. Pharmacol Biochem Behav. 1987;27:391–398. - PubMed
- Braida D, Iosue S, Pegorini S, Sala M. Delta-9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004a;506:63–69. - PubMed
- Braida D, Iosue S, Pegorini S, Sala M. Delta-9-tetrahydrocannabinol induced conditioned place preference and intracerebroventricular self administration in rats. Eur J Pharmacol. 2004b;506:63–69. - PubMed
- Braida D, Pozzi M, Parolaro D, Sala M. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur J Pharmacol. 2001;413:227–234. - PubMed
- Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000;295:328–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA003672/DA/NIDA NIH HHS/United States
- DA-03672/DA/NIDA NIH HHS/United States
- DA-031988/DA/NIDA NIH HHS/United States
- R03 DA031988/DA/NIDA NIH HHS/United States
- R37 DA003672/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous