Bacterial collagen-like proteins that form triple-helical structures - PubMed (original) (raw)
Bacterial collagen-like proteins that form triple-helical structures
Zhuoxin Yu et al. J Struct Biol. 2014 Jun.
Abstract
A large number of collagen-like proteins have been identified in bacteria during the past 10years, principally from analysis of genome databases. These bacterial collagens share the distinctive Gly-Xaa-Yaa repeating amino acid sequence of animal collagens which underlies their unique triple-helical structure. A number of the bacterial collagens have been expressed in Escherichia coli, and they all adopt a triple-helix conformation. Unlike animal collagens, these bacterial proteins do not contain the post-translationally modified amino acid, hydroxyproline, which is known to stabilize the triple-helix structure and may promote self-assembly. Despite the absence of collagen hydroxylation, the triple-helix structures of the bacterial collagens studied exhibit a high thermal stability of 35-39°C, close to that seen for mammalian collagens. These bacterial collagens are readily produced in large quantities by recombinant methods, either in the original amino acid sequence or in genetically manipulated sequences. This new family of recombinant, easy to modify collagens could provide a novel system for investigating structural and functional motifs in animal collagens and could also form the basis of new biomedical materials with designed structural properties and functions.
Keywords: Biomedical material; Collagen; Prokaryote; Recombinant expression; Thermal stability; Triple-helix.
Copyright © 2014. Published by Elsevier Inc.
Figures
Fig. 1
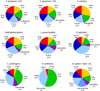
A pie chart representation of the non-Gly amino acid composition of bacterial collagen-like domains. Only the amino acids at Xaa and Yaa positions were taken into consideration. The groups include: hydrophobic (Val, Ile, Leu, Met, Phe), polar (Ser, Thr, Asn, Gln), charged (Asp, Glu, Lys, Arg, His), small (Gly and Ala) and Pro. The amino acid composition of the α1 chain of human4type I collagens is shown for comparison.
Fig. 2
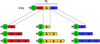
A diagram of the constructs including A, B, or C fragments of the S. pyogenes Scl2 CL domain, showing the rod-like CL domain, the globular V domain, and a small diamond to represent the His6-tag at the N terminus. Fragments A, B and C were expressed individually in E. coli, or fused with the V domain to promote trimerization. Each fragment was also tandemly fused to construct a dimer (AA, BB, or CC) or a trimer (AAA, BBB, or CCC) appended to the V domain. Although CCC was successfully expressed it was insoluble (Yu et al. 2011
Fig. 3
A diagram illustrating various substitutions and insertions of functional domains from animal collagens that have been made in S. pyogenes Scl2 constructs, including integrin (Seo et al. 2010; An et al. 2013; Peng et al. 2013); heparin (Peng et al. 2013); fibronectin (An et al. 2013), and MMP-1 (Yu et al. 2012).
Similar articles
- Expanding the family of collagen proteins: recombinant bacterial collagens of varying composition form triple-helices of similar stability.
Xu C, Yu Z, Inouye M, Brodsky B, Mirochnitchenko O. Xu C, et al. Biomacromolecules. 2010 Feb 8;11(2):348-56. doi: 10.1021/bm900894b. Biomacromolecules. 2010. PMID: 20025291 Free PMC article. - Noncollagenous region of the streptococcal collagen-like protein is a trimerization domain that supports refolding of adjacent homologous and heterologous collagenous domains.
Yu Z, Mirochnitchenko O, Xu C, Yoshizumi A, Brodsky B, Inouye M. Yu Z, et al. Protein Sci. 2010 Apr;19(4):775-85. doi: 10.1002/pro.356. Protein Sci. 2010. PMID: 20162611 Free PMC article. - Bioengineered collagens: emerging directions for biomedical materials.
Ramshaw JA, Werkmeister JA, Dumsday GJ. Ramshaw JA, et al. Bioengineered. 2014 Jul-Aug;5(4):227-33. doi: 10.4161/bioe.28791. Epub 2014 Apr 9. Bioengineered. 2014. PMID: 24717980 Free PMC article. Review. - Hydroxyproline Ring Pucker Causes Frustration of Helix Parameters in the Collagen Triple Helix.
Chow WY, Bihan D, Forman CJ, Slatter DA, Reid DG, Wales DJ, Farndale RW, Duer MJ. Chow WY, et al. Sci Rep. 2015 Jul 29;5:12556. doi: 10.1038/srep12556. Sci Rep. 2015. PMID: 26220399 Free PMC article. - Bioengineered Collagens.
Brodsky B, Ramshaw JA. Brodsky B, et al. Subcell Biochem. 2017;82:601-629. doi: 10.1007/978-3-319-49674-0_18. Subcell Biochem. 2017. PMID: 28101874 Review.
Cited by
- Comparative Pangenomics of the Mammalian Gut Commensal Bifidobacterium longum.
Albert K, Rani A, Sela DA. Albert K, et al. Microorganisms. 2019 Dec 18;8(1):7. doi: 10.3390/microorganisms8010007. Microorganisms. 2019. PMID: 31861401 Free PMC article. - Preparation and characterization of monomers to tetramers of a collagen-like domain from Streptococcus pyogenes.
Peng YY, Stoichevska V, Howell L, Madsen S, Werkmeister JA, Dumsday GJ, Ramshaw JA. Peng YY, et al. Bioengineered. 2014;5(6):378-85. doi: 10.4161/21655979.2014.969168. Epub 2014 Nov 11. Bioengineered. 2014. PMID: 25482084 Free PMC article. - A Significantly High Abundance of "Candidatus Liberibacter asiaticus" in Citrus Fruit Pith: in planta Transcriptome and Anatomical Analyses.
Fang F, Guo H, Zhao A, Li T, Liao H, Deng X, Xu M, Zheng Z. Fang F, et al. Front Microbiol. 2021 Jun 11;12:681251. doi: 10.3389/fmicb.2021.681251. eCollection 2021. Front Microbiol. 2021. PMID: 34177866 Free PMC article. - Synthesis and Application of Collagens for Assembling a Corneal Implant.
Edin E, Simpson F, Griffith M. Edin E, et al. Methods Mol Biol. 2020;2145:169-183. doi: 10.1007/978-1-0716-0599-8_12. Methods Mol Biol. 2020. PMID: 32542607 - Genome-Wide Association Analysis Identifies a Genetic Basis of Infectivity in a Model Bacterial Pathogen.
Andras JP, Fields PD, Du Pasquier L, Fredericksen M, Ebert D. Andras JP, et al. Mol Biol Evol. 2020 Dec 16;37(12):3439-3452. doi: 10.1093/molbev/msaa173. Mol Biol Evol. 2020. PMID: 32658956 Free PMC article.
References
- Adachi E, Katsumato O, Yamashina S, Prockop DJ, Fertala A. Collagen II containing a Cys substitution for Arg-alpha1-519. Analysis by atomic force microscopy demonstrates that mutated monomers alter the topography of the surface of collagen II fibrils. Matrix Biol. 1999;18:189–196. - PubMed
- Arnold WV, Fertala A, Sieron AL, Hattori H, Mechling D, Bächinger HP, Prockop DJ. Recombinant procollagen II: Deletion of D period segments identifies sequences that are required for helix stabilization and generates a temperature-sensitive N-proteinase cleavage site. J. Biol. Chem. 1998;273:31822–31828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous