Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors - PubMed (original) (raw)
Multicenter Study
Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors
Xin Shelley Wang et al. Cancer. 2014.
Abstract
Background: The effective management of fatigue in patients with cancer requires a clear delineation of what constitutes nontrivial fatigue. The authors defined numeric cutpoints for fatigue severity based on functional interference and described the prevalence and characteristics of fatigue in patients with cancer and survivors.
Methods: In a multicenter study, outpatients with breast, prostate, colorectal, or lung cancer rated their fatigue severity and symptom interference with functioning on the M. D. Anderson Symptom Inventory numeric scale of 0 to 10. Ratings of symptom interference guided the selection of numeric rating cutpoints between mild, moderate, and severe fatigue levels. Regression analysis identified significant factors related to reporting moderate=severe fatigue .
Results: The statistically optimal cutpoints were 4 for moderate fatigue and 7 for severe fatigue. Moderate=severe fatigue was reported by 983 of 2177 patients (45%) undergoing active treatment and was more likely to occur in patients receiving treatment with strong opioids (odds ratio [OR], 3.00), those with a poor Eastern Cooperative Oncology Group performance status (OR, 2.00), those who had >5% weight loss within 6 months (OR, 1.60), those who were receiving >10 medications (OR, 1.58), those with lung cancer (OR, 1.55), and those with a history of depression (OR, 1.42). Among survivors (patients with complete remission or no evidence of disease, and not currently receiving cancer treatment), 29% of patients (150 of 515 patients) had moderate=severe fatigue that was associated with poor performance status (OR, 3.48) and a history of depression (OR, 2.21).
Conclusions: The current study statistically defined fatigue severity categories related to significantly increased symptom interference. The high prevalence of moderate=severe fatigue in both actively treated patients with cancer and survivors warrants the promoting of the routine assessment and management of patient-reported fatigue.
Figures
Figure 1
Interaction of Symptom Interference and Fatigue Severity Using the Identified Optimal Cutpoints. MDASI indicates M. D. Anderson Symptom Inventory.
Figure 2
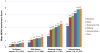
Prevalence of fatigue by cancer type (M. D. Anderson Symptom Inventory ratings) A: Patients Undergoing Treatment vs Survivors Significant differences were found between patients undergoing treatment and survivors in terms of the percentages reporting moderate-to-severe fatigue: P = .005 for breast cancer (40% of treated patients vs 31% of survivors), P < .001 for colorectal cancer (46% vs 27%), _P_ = .001 for prostate cancer (49% vs 22%), and _P_ < .001 for lung cancer (59% vs 33%), using the Fisher exact test. In patients undergoing treatment, significant differences were found in the percentages of those reporting moderate-to-severe fatigue in pairwise comparisons between lung cancer and other cancer types: _P_ < .001 for breast cancer (59% lung vs 40% breast), _P_ < .001 for colorectal cancer (59% vs 46%), and _P_ = .018 for prostate cancer (59% vs 49%), using the Chi square test. Fisher exact test results were not significant for pairwise comparisons of moderate-to-severe fatigue between lung cancer and other cancer types in survivors (_P_ > .05 for all comparisons). B: Short-Term Survivors vs Long-Term Survivors For comparisons between the percentages of short-term survivors and long-term survivors reporting moderate-to-severe fatigue, P = .001 for breast cancer (38% short-term vs 18% long-term), P = .067 for colorectal cancer (23% vs 43%), no significance for prostate cancer (23% vs 18%), and for lung cancer (35% vs 0%), using the Fisher exact test. Six patients had a missing value for time since cancer diagnosis and were not included in this analysis.
Figure 2
Prevalence of fatigue by cancer type (M. D. Anderson Symptom Inventory ratings) A: Patients Undergoing Treatment vs Survivors Significant differences were found between patients undergoing treatment and survivors in terms of the percentages reporting moderate-to-severe fatigue: P = .005 for breast cancer (40% of treated patients vs 31% of survivors), P < .001 for colorectal cancer (46% vs 27%), _P_ = .001 for prostate cancer (49% vs 22%), and _P_ < .001 for lung cancer (59% vs 33%), using the Fisher exact test. In patients undergoing treatment, significant differences were found in the percentages of those reporting moderate-to-severe fatigue in pairwise comparisons between lung cancer and other cancer types: _P_ < .001 for breast cancer (59% lung vs 40% breast), _P_ < .001 for colorectal cancer (59% vs 46%), and _P_ = .018 for prostate cancer (59% vs 49%), using the Chi square test. Fisher exact test results were not significant for pairwise comparisons of moderate-to-severe fatigue between lung cancer and other cancer types in survivors (_P_ > .05 for all comparisons). B: Short-Term Survivors vs Long-Term Survivors For comparisons between the percentages of short-term survivors and long-term survivors reporting moderate-to-severe fatigue, P = .001 for breast cancer (38% short-term vs 18% long-term), P = .067 for colorectal cancer (23% vs 43%), no significance for prostate cancer (23% vs 18%), and for lung cancer (35% vs 0%), using the Fisher exact test. Six patients had a missing value for time since cancer diagnosis and were not included in this analysis.
References
- Yanez B, Pearman T, Lis CG, Beaumont JL, Cella D. The FACT-G7: a rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol. 2013;24(4):1073–1078. - PubMed
- Minton O, Strasser F, Radbruch L, Stone P. Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage. 2012;43(2):226–235. - PubMed
- Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. - PubMed
- Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology. 2007;16(9):787–795. - PubMed
Publication types
MeSH terms
Grants and funding
- P30CA016672/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- R01CA026582/CA/NCI NIH HHS/United States
- U10 CA037403/CA/NCI NIH HHS/United States
- U10 CA17145/CA/NCI NIH HHS/United States
- R01 CA026582/CA/NCI NIH HHS/United States
- U10 CA37403/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical