Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas - PubMed (original) (raw)
Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas
Daniel Schramek et al. Science. 2014.
Abstract
Mining modern genomics for cancer therapies is predicated on weeding out "bystander" alterations (nonconsequential mutations) and identifying "driver" mutations responsible for tumorigenesis and/or metastasis. We used a direct in vivo RNA interference (RNAi) strategy to screen for genes that upon repression predispose mice to squamous cell carcinomas (SCCs). Seven of our top hits-including Myh9, which encodes nonmuscle myosin IIa-have not been linked to tumor development, yet tissue-specific Myh9 RNAi and Myh9 knockout trigger invasive SCC formation on tumor-susceptible backgrounds. In human and mouse keratinocytes, myosin IIa's function is manifested not only in conventional actin-related processes but also in regulating posttranscriptional p53 stabilization. Myosin IIa is diminished in human SCCs with poor survival, which suggests that in vivo RNAi technology might be useful for identifying potent but low-penetrance tumor suppressors.
Figures
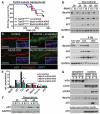
Fig. 1. Direct in vivo shRNA screen for HNSCC tumor suppressors
(A) Schematic of pooled shRNA screen. (B) Tumor-free survival of mice of the indicated genotype transduced at E9.5 with the shRNA library targeting putative HNSCC genes (n = number per group; P < 0.0001, log-rank test). (C) Representative pie charts showing percent representation of a particular shRNA within an individual tumor compared to surrounding healthy skin. (D) Top-scoring tumor suppressor candidates and corresponding numbers of tumors showing significant enrichment.
Fig. 2. Functional validation of Myh9 as a bona fide tumor suppressor
(A) Tumor-free survival of mice of the indicated genotype and shRNA transduction (n > 6 for each genotype, P < 0.0001). Insert shows skin lesions (arrows) on 4-month-old Myh9 shRNA–transduced _T_β_RII_-cKO mouse. (B) Myh9 knockdown results in pulmonary metastases in _T_β_RII_-cKO mice. Metastatic lesions are immunoreactive for epithelial keratin 14 and negative for myosin IIa. (C) Tumor-free survival of Myh9/T_β_RII inducible knockout (iKO) as well as Myh9 heterozygous/T_β_RII iKO and control mice (n = 6, P < 0.001, log-rank test).
Fig. 3. A noncanonical role for myosin IIa in nuclear retention of activated p53
(A) Tumor-free survival of conditional Trp53 mutant mice transduced with the indicated shRNA (n > 6 for each genotype; no significant survival change could be observed). (B) Myh9 knockdown (kd) but not scrambled control shRNA (c) diminishes p53 and targets p21 (CDKN2) levels in response to the DDR inducer doxorubicin. Myosin IIa and GAPDH levels are shown as controls. (C and D) Lack of nuclear p53 in _Myh9_-cKO versus control (Ctrl) littermate skins 6 hours after γ irradiation (5 Gy). (C) Immunofluorescence (boxed regions show DAPI-stained nuclei in blue); (D) immunoblot analysis. Myosin IIa and GAPDH levels are shown as controls. (E) Quantitative polymerase chain reaction of p53 target genes illustrates the effects of Myh9 knockdown on the p53 pathway. (F) p53 immunoblot of lysates from DDR-induced keratinocytes treated with vehicle (V), blebbistatin (B), Rho kinase inhibitor Y27632 (Y), or latrunculin B (L). GAPDH levels are shown as controls. (G) Nuclear p53 is not retained when DDR-induced Myh9 knockdown primary keratinocytes are exposed to blebbistatin (B). Lamin A/C, IκBα, and γH2AX are controls for nuclear fraction, cytoplasmic fraction, and DDR, respectively. Nuclear export inhibitor leptomycin B rescues the ability of _Myh9_-deficient cells to retain p53 in the nucleus.
Fig. 4. MYH9 is a bona fide tumor suppressor in human HNSCC
(A) p53 induction in primary human keratinocytes treated with the myosin ATPase inhibitor blebbistatin and with the DDR inducer doxorubicin. GAPDH levels are shown as loading control. (B) Myosin IIa quantifications on 362 samples of human healthy skin, skin SCCs, and HNSCCs. A substantial fraction of cases show absent or reduced myosin IIa expression. (C) Decreased MYH9 mRNA expression correlates with shortened survival. Kaplan-Meier analysis compares overall survival of TCGA HNSCC patients stratified according to the lowest (≤5th percentile) MYH9 expression versus the rest (>5th percentile) (n = 166, P = 0.0044, log-rank test).
Similar articles
- Nonmuscle myosin IIA is associated with poor prognosis of esophageal squamous cancer.
Xia ZK, Yuan YC, Yin N, Yin BL, Tan ZP, Hu YR. Xia ZK, et al. Dis Esophagus. 2012 Jul;25(5):427-36. doi: 10.1111/j.1442-2050.2011.01261.x. Epub 2011 Sep 23. Dis Esophagus. 2012. PMID: 21951916 - MYH9 regulates p53 stability and is a tumor suppressor in SCC.
[No authors listed] [No authors listed] Cancer Discov. 2014 Mar;4(3):OF22. doi: 10.1158/2159-8290.CD-RW2014-023. Epub 2014 Jan 30. Cancer Discov. 2014. PMID: 24596217 - The tumor suppressor capability of p53 is dependent on non-muscle myosin IIA function in head and neck cancer.
Coaxum SD, Tiedeken J, Garrett-Mayer E, Myers J, Rosenzweig SA, Neskey DM. Coaxum SD, et al. Oncotarget. 2017 Apr 4;8(14):22991-23007. doi: 10.18632/oncotarget.14967. Oncotarget. 2017. PMID: 28160562 Free PMC article. - Myosin Heavy Chain 9: Oncogene or Tumor Suppressor Gene?
Wang Y, Liu S, Zhang Y, Yang J. Wang Y, et al. Med Sci Monit. 2019 Jan 31;25:888-892. doi: 10.12659/MSM.912320. Med Sci Monit. 2019. PMID: 30739906 Free PMC article. Review. - Non-Muscle Myosin II A: Friend or Foe in Cancer?
Feroz W, Park BS, Siripurapu M, Ntim N, Kilroy MK, Sheikh AMA, Mishra R, Garrett JT. Feroz W, et al. Int J Mol Sci. 2024 Aug 30;25(17):9435. doi: 10.3390/ijms25179435. Int J Mol Sci. 2024. PMID: 39273383 Free PMC article. Review.
Cited by
- Myosin light chain kinase regulates cell polarization independently of membrane tension or Rho kinase.
Lou SS, Diz-Muñoz A, Weiner OD, Fletcher DA, Theriot JA. Lou SS, et al. J Cell Biol. 2015 Apr 27;209(2):275-88. doi: 10.1083/jcb.201409001. J Cell Biol. 2015. PMID: 25918227 Free PMC article. - ADCK2 Knockdown Affects the Migration of Melanoma Cells via MYL6.
Vierthaler M, Sun Q, Wang Y, Steinfass T, Poelchen J, Hielscher T, Novak D, Umansky V, Utikal J. Vierthaler M, et al. Cancers (Basel). 2022 Feb 20;14(4):1071. doi: 10.3390/cancers14041071. Cancers (Basel). 2022. PMID: 35205819 Free PMC article. - Multiple S100 protein isoforms and C-terminal phosphorylation contribute to the paralog-selective regulation of nonmuscle myosin 2 filaments.
Ecsédi P, Billington N, Pálfy G, Gógl G, Kiss B, Bulyáki É, Bodor A, Sellers JR, Nyitray L. Ecsédi P, et al. J Biol Chem. 2018 Sep 21;293(38):14850-14867. doi: 10.1074/jbc.RA118.004277. Epub 2018 Aug 7. J Biol Chem. 2018. PMID: 30087119 Free PMC article. - HMGA1 stimulates MYH9-dependent ubiquitination of GSK-3β via PI3K/Akt/c-Jun signaling to promote malignant progression and chemoresistance in gliomas.
Que T, Zheng H, Zeng Y, Liu X, Qi G, La Q, Liang T, Li Z, Yi G, Zhang S, Li J, Nie J, Tan JE, Huang G. Que T, et al. Cell Death Dis. 2021 Dec 10;12(12):1147. doi: 10.1038/s41419-021-04440-x. Cell Death Dis. 2021. PMID: 34887392 Free PMC article. - Preclinical Advances with Multiphoton Microscopy in Live Imaging of Skin Cancers.
Sun TY, Haberman AM, Greco V. Sun TY, et al. J Invest Dermatol. 2017 Feb;137(2):282-287. doi: 10.1016/j.jid.2016.08.033. Epub 2016 Nov 12. J Invest Dermatol. 2017. PMID: 27847119 Free PMC article. Review.
References
- Leemans CR, Braakhuis BJ, Brakenhoff RH. Nat. Rev. Cancer. 2011;11:9–22. - PubMed
- Berton TR, et al. Oncogene. 2003;22:5415–5426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous