AMPK agonist AICAR improves cognition and motor coordination in young and aged mice - PubMed (original) (raw)
AMPK agonist AICAR improves cognition and motor coordination in young and aged mice
Tali Kobilo et al. Learn Mem. 2014.
Abstract
Normal aging can result in a decline of memory and muscle function. Exercise may prevent or delay these changes. However, aging-associated frailty can preclude physical activity. In young sedentary animals, pharmacological activation of AMP-activated protein kinase (AMPK), a transcriptional regulator important for muscle physiology, enhanced spatial memory function, and endurance. In the present study we investigated effects of AMPK agonist 5-aminoimidazole-4-carboxamide riboside (AICAR) on memory and motor function in young (5- to 7-wk-old) and aged (23-mo-old) female C57Bl/6 mice, and in young (4- to 6-wk-old) transgenic mice with muscle-specific mutated AMPK α2-subunit (AMPK-DN). Mice were injected with AICAR (500 mg/kg) for 3-14 d. Two weeks thereafter animals were tested in the Morris water maze, rotarod, and open field. Improved water maze performance and motor function were observed, albeit at longer duration of administration, in aged (14-d AICAR) than in young (3-d AICAR) mice. In the AMPK-DN mice, the compound did not enhance behavior, providing support for a muscle-mediated mechanism. In addition, microarray analysis of muscle and hippocampal tissue derived from aged mice treated with AICAR revealed changes in gene expression in both tissues, which correlated with behavioral effects in a dose-dependent manner. Pronounced up-regulation of mitochondrial genes in muscle was observed. In the hippocampus, genes relevant to neuronal development and plasticity were enriched. Altogether, endurance-related factors may mediate both muscle and brain health in aging, and could play a role in new therapeutic interventions.
Figures
Figure 1.
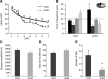
Water maze performance and motor behavior in mice treated with saline (CON) or AICAR (ACR3, 500 mg/kg) for 3 d. Mice were trained for 7 d with four trials per day in the Morris water maze, 2 wk after injections. (A) ACR3 mice had a trend of shorter latency to the platform over training days than CON mice (P = 0.08). (B) Only the ACR3 mice showed a significant preference for the target zone as compared to all other quadrants of the pool in the 4-h probe trial (*P < 0.006). (C–E) To assess motor behavior mice were tested in the open field and rotarod. (C) Distance traveled in the open field showed a modest trend toward reduction for ACR injected mice. (D) Latency to the first fall in the rotarod did not differ between the groups. (E) In the rotarod the ACR3 group had significantly less total falls (*P < 0.015). Error bars indicate SEM.
Figure 2.
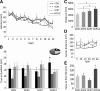
Water maze and motor behavior in aged C57Bl/6 mice (23-mo old) treated with AICAR for 3 (ACR3), 7 (ACR7) or 14 (ACR14) d or injected with saline (CON). (A) Mice were tested in the Morris water maze with four trials per day for 12 d. There was no difference between the groups in acquisition of the task (P > 0.65). (B) In the probe trial conducted 4 h after the last training trial, ACR14 mice showed a clear preference for the target quadrant as compared to all other quadrants (*P < 0.03). (C) Locomotor activity was examined in the open field arena over 30 min. ACR7 and ACR14 mice traveled a greater distance in the open field than CON mice, and ACR14 also differed significantly from ACR3 (*P < 0.05). (D) Distance traveled in the center of the open field did not differ between the groups. (E) Motor coordination and strength were tested in the rotarod. Mice injected with AICAR for 14 d showed a longer latency to the first fall than the other groups (*P < 0.02). Error bars indicate SEM.
Figure 3.
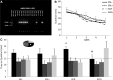
Effect of AICAR on cognition in AMPK-DN mice. (A) PCR analysis of isolated tail DNA was performed to identify AMPK-DN (tg) or wt mice. (B) Water maze performance in AMPK-DN mice (+) or wild type mice (−) treated with Saline (SAL) or AICAR (ACR, 500 mg/kg) for 3 d. Mice were trained for 5 d with four trials per day in the Morris water maze, 2 wk after injections. There was no difference between the groups in acquisition of the task. (C) In the probe trial 4 h after the last training trial drug-treated wt mice that were injected with ACR (ACR−) showed a significant preference for the platform quadrant compared to the other quadrants (*P < 0.03). The mice treated with saline (wt, SAL−, and tg, SAL+) and the tg mice treated with AICAR (ACR+) showed no retention of platform location. In addition, there was a trend toward a significant difference in time spent in the target quadrant between ACR− and ACR+ mice (#P = 0.06), further supporting memory retention enhancement by AICAR in wt mice. Error bars indicate SEM.
Figure 4.
Microarray analysis of hippocampus and muscle tissue derived from aged mice treated with saline (CON) or AICAR for 3 d (ACR3), 7 d (ACR7), or 14 d (ACR14). (A) Gene set analysis of gastrocnemius tissue. Increased expression of mitochondrial inner membrane and metabolism related genes was observed. Each section represents the number of up-regulated genes of the respective function. (B) The heat-map illustrates the expression of the selected signature genes derived from microarray analysis. Up-regulated genes are colored in red and down-regulated genes are colored in green. (C) Top up-regulated and down-regulated genes by ACR7 and ACR14 in gastrocnemius. The tables list the most highly up-regulated genes related to energy metabolism and neuronal development; up-regulation is expressed as fold change compared to control and as _z_-ratio for each gene. (D) Top up-regulated and down-regulated genes by ACR7 and ACR14 in hippocampus. The tables list the most highly up-regulated genes related to energy metabolism and neuronal development; up-regulation is expressed as fold change compared to control and as _z_-ratio for each gene. (E) Relative gene expression level and validation. qPCR analysis of expression levels of four genes in ACR14 samples compared to CON, in gastrocnemius. (F) Relative gene expression level and validation. qPCR analysis of expression levels of four genes in ACR14 samples compared to CON, in hippocampus. N = 4 per group. Error bars indicate SEM.
Similar articles
- Blunted response of hippocampal AMPK associated with reduced neurogenesis in older versus younger mice.
Jang S, Kim H, Jeong J, Lee SK, Kim EW, Park M, Kim CH, Lee JE, Namkoong K, Kim E. Jang S, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Nov 3;71:57-65. doi: 10.1016/j.pnpbp.2016.06.011. Epub 2016 Jun 22. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 27343360 - α2 isoform-specific activation of 5'adenosine monophosphate-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-β-D-ribonucleoside at a physiological level activates glucose transport and increases glucose transporter 4 in mouse skeletal muscle.
Nakano M, Hamada T, Hayashi T, Yonemitsu S, Miyamoto L, Toyoda T, Tanaka S, Masuzaki H, Ebihara K, Ogawa Y, Hosoda K, Inoue G, Yoshimasa Y, Otaka A, Fushiki T, Nakao K. Nakano M, et al. Metabolism. 2006 Mar;55(3):300-8. doi: 10.1016/j.metabol.2005.09.003. Metabolism. 2006. PMID: 16483872 - Endurance factors improve hippocampal neurogenesis and spatial memory in mice.
Kobilo T, Yuan C, van Praag H. Kobilo T, et al. Learn Mem. 2011 Jan 18;18(2):103-7. doi: 10.1101/lm.2001611. Print 2011 Feb. Learn Mem. 2011. PMID: 21245211 Free PMC article. - Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction.
Kim DM, Leem YH. Kim DM, et al. Neuroscience. 2016 Jun 2;324:271-85. doi: 10.1016/j.neuroscience.2016.03.019. Epub 2016 Mar 11. Neuroscience. 2016. PMID: 26975895 - Enhancement of physiology via adaptive transcription.
Lissek T. Lissek T. Pflugers Arch. 2024 Nov 1. doi: 10.1007/s00424-024-03037-5. Online ahead of print. Pflugers Arch. 2024. PMID: 39482558 Review.
Cited by
- Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer's disease.
Zhao R. Zhao R. J Neuroinflammation. 2024 Feb 2;21(1):40. doi: 10.1186/s12974-024-03031-9. J Neuroinflammation. 2024. PMID: 38308368 Free PMC article. Review. - Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer's disease.
Velazquez R, Tran A, Ishimwe E, Denner L, Dave N, Oddo S, Dineley KT. Velazquez R, et al. Neurobiol Aging. 2017 Oct;58:1-13. doi: 10.1016/j.neurobiolaging.2017.06.003. Epub 2017 Jun 17. Neurobiol Aging. 2017. PMID: 28688899 Free PMC article. - Can exercise benefits be harnessed with drugs? A new way to combat neurodegenerative diseases by boosting neurogenesis.
Zhao R. Zhao R. Transl Neurodegener. 2024 Jul 25;13(1):36. doi: 10.1186/s40035-024-00428-7. Transl Neurodegener. 2024. PMID: 39049102 Free PMC article. Review. - AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review.
Višnjić D, Lalić H, Dembitz V, Tomić B, Smoljo T. Višnjić D, et al. Cells. 2021 May 4;10(5):1095. doi: 10.3390/cells10051095. Cells. 2021. PMID: 34064363 Free PMC article. - Role of physical and mental training in brain network configuration.
Foster PP. Foster PP. Front Aging Neurosci. 2015 Jun 23;7:117. doi: 10.3389/fnagi.2015.00117. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26157387 Free PMC article. Review.
References
- Avraham H, Park SY, Schinkmann K, Avraham S 2000. RAFTK/Pyk2-mediated cellular signaling. Cell Signal 12: 123–133 - PubMed
- Baumgartner RN, Rhyne RL, Troup C, Wayne S, Garry PJ 1992. Appendicular skeletal muscle areas assessed by magnetic resonance imaging in older persons. J Gerontol 47: M67–M72 - PubMed
- Bratic I, Trifunovic A 2010. Mitochondrial energy metabolism and ageing. Biochim Biophys Acta 1797: 961–967 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical