Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice - PubMed (original) (raw)
Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice
Jianquan Chen et al. PLoS One. 2014.
Abstract
Osterix (Osx or Sp7) is a zinc-finger-family transcriptional factor essential for osteoblast differentiation in mammals. The Osx-Cre mouse line (also known as Osx1-GFP::Cre) expresses GFP::Cre fusion protein from a BAC transgene containing the Osx regulatory sequence. The mouse strain was initially characterized during embryogenesis, and found to target mainly osteoblast-lineage cells. Because the strain has been increasingly used in postnatal studies, it is important to evaluate its targeting specificity in mice after birth. By crossing the Osx-Cre mouse with the R26-mT/mG reporter line and analyzing the progenies at two months of age, we find that Osx-Cre targets not only osteoblasts, osteocytes and hypertrophic chondrocytes as expected, but also stromal cells, adipocytes and perivascular cells in the bone marrow. The targeting of adipocytes and perivascular cells appears to be specific to those residing within the bone marrow, as the same cell types elsewhere are not targeted. Beyond the skeleton, Osx-Cre also targets the olfactory glomerular cells, and a subset of the gastric and intestinal epithelium. Thus, potential contributions from the non-osteoblast-lineage cells should be considered when Osx-Cre is used to study gene functions in postnatal mice.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
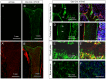
Figure 1. Osx-Cre targets osteoblast lineage cells, hypertrophic chondrocytes and bone marrow stromal cells.
(A–B) Confocal images for direct fluorescence from EGFP (A, B) or EGFP/tdTomato (A′, B′) on longitudinal tibial sections from two-month-old R26-mT/mG (A, A′) or Osx-Cre; R26-mT/mG mice (B, B′); 1: chondro-osseous junction of primary ossification center; 2: secondary ossification center. (C–F, C′–F′) Higher magnification images for EGFP (C–F) or EGFP/tdTomato: trabecular bone (C, C′), cortical bone (D, D′), growth plate (E, E′) and bone marrow (F, F′). Red: membrane-targeted tdTomato; Green: membrane-targeted EGFP; Blue: DAPI. Arrow: osteocyte.
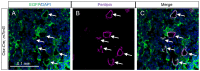
Figure 2. Osx-Cre marks adipocytes in bone marrow.
(A–B) Double immunostaining for EGFP (A) and perilipin (B) on longitudinal sections of tibias from two-month-old Osx-Cre; R26-mT/mG mice. (C) Co-localization of EGFP and perilipin. Arrows denote co-expression of GFP and perilipin. Green: EGFP; magenta: perilipin; blue: DAPI.
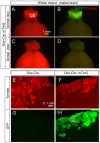
Figure 3. Osx-Cre does not mark non-bone marrow adipocytes.
(A–B) Images for direct fluorescence from tdTomato (A) or EGFP (B) in whole-mount gonadal fat depots from two-month-old Osx-Cre; R26-mT/mG mice. (C–F) Direct fluorescence for tdTomato (C) and immunofluorescence for perilipin (D) and EGFP (E) on sections of gonadal fat depots from two-month-old Osx-Cre;R26-mT/mG mice. (G–K) Imaging of longitudinal sections of an intramuscular fat depot associated with a tibia from two-month-old Osx-Cre;R26-mT/mG mice. G: perilipin immunofluorescence; H: EGFP immunofluorescence; I: direct fluorescence for tdTomato; J: merged view of G–I; K: DAPI staining. Arrow: GFP-positive periosteum.
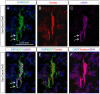
Figure 4. Osx-Cre marks perivascular smooth muscle cells in bone marrow.
(A–C) Confocal images of EGFP (A), tdTomato (B), and αSMA (C) on longitudinal sections of tibias from two-month-old Osx-Cre; R26-mT/mG mice. (D–F) Merged images. Arrow: co-expression of EGFP and αSMA. EGFP and αSMA: immunofluorescence; tdTomato: direct fluorescence. DAPI stains DNA blue.
Figure 5. Osx-Cre targets olfactory glomerular cells.
(A–D) Direct fluorescence of whole-mount rostral brain from a two-month-old Osx-Cre; R26-mT/mG mouse. ob: olfactory bulb. (E–H) Direct fluorescence of tdTomato (E, F) and EGFP (G, H) on sagittal sections through the olfactory bulb of Osx-Cre (E, G) or Osx-Cre; R26-mT/mG (F, H) mice at two months of age. GL: glomerular layer.
Figure 6. Osx-Cre targets gastric and intestinal epithelia.
(A–D) Direct fluorescence of tdTomato (A, C) and EGFP (B, D) in whole-mount small intestine of two-month-old R26-mT/mG (A, B) or Osx-Cre; R26-mT/mG mice (C, D). (E–G) Direct fluorescence on cross sections of small intestine from a two-month-old Osx-Cre; R26-mT/mG mouse. Red-boxed area in F shown at a higher magnification in G. (H–J) Direct fluorescence on stomach sections from a two-month-old Osx-Cre; R26-mT/mG mouse. Red-boxed area in I shown at a higher magnification in J. White arrow denotes parietal cells.
Similar articles
- Use of green fluorescent fusion protein to track activation of the transcription factor osterix during early osteoblast differentiation.
Tai G, Christodoulou I, Bishop AE, Polak JM. Tai G, et al. Biochem Biophys Res Commun. 2005 Aug 12;333(4):1116-22. doi: 10.1016/j.bbrc.2005.05.195. Biochem Biophys Res Commun. 2005. PMID: 15979565 - IGF-I Signaling in Osterix-Expressing Cells Regulates Secondary Ossification Center Formation, Growth Plate Maturation, and Metaphyseal Formation During Postnatal Bone Development.
Wang Y, Menendez A, Fong C, ElAlieh HZ, Kubota T, Long R, Bikle DD. Wang Y, et al. J Bone Miner Res. 2015 Dec;30(12):2239-48. doi: 10.1002/jbmr.2563. Epub 2015 Jul 29. J Bone Miner Res. 2015. PMID: 26011431 Free PMC article. - Postnatally induced inactivation of Osterix in osteoblasts results in the reduction of bone formation and maintenance.
Baek WY, de Crombrugghe B, Kim JE. Baek WY, et al. Bone. 2010 Apr;46(4):920-8. doi: 10.1016/j.bone.2009.12.007. Epub 2009 Dec 21. Bone. 2010. PMID: 20026264 Free PMC article. - Molecular mechanisms of osteoblast-specific transcription factor Osterix effect on bone formation.
Zhang C. Zhang C. Beijing Da Xue Xue Bao Yi Xue Ban. 2012 Oct 18;44(5):659-65. Beijing Da Xue Xue Bao Yi Xue Ban. 2012. PMID: 23073571 Review. - Genetic and molecular control of osterix in skeletal formation.
Sinha KM, Zhou X. Sinha KM, et al. J Cell Biochem. 2013 May;114(5):975-84. doi: 10.1002/jcb.24439. J Cell Biochem. 2013. PMID: 23225263 Free PMC article. Review.
Cited by
- Loss of Vhl alters trabecular bone loss during S. aureus osteomyelitis in a cell-specific manner.
Ford CA, Hurford IM, Fulbright LE, Curry JM, Peek CT, Spoonmore TJ, Cruz Victorio V, Johnson JR, Peck SH, Cassat JE. Ford CA, et al. Front Cell Infect Microbiol. 2022 Sep 20;12:985467. doi: 10.3389/fcimb.2022.985467. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36204648 Free PMC article. - Genetic determination of the cellular basis of the ghrelin-dependent bone remodeling.
Ma C, Fukuda T, Ochi H, Sunamura S, Xu C, Xu R, Okawa A, Takeda S. Ma C, et al. Mol Metab. 2015 Jan 21;4(3):175-85. doi: 10.1016/j.molmet.2015.01.002. eCollection 2015 Mar. Mol Metab. 2015. PMID: 25737953 Free PMC article. - WWOX promotes osteosarcoma development via upregulation of Myc.
Akkawi R, Hidmi O, Haj-Yahia A, Monin J, Diment J, Drier Y, Stein GS, Aqeilan RI. Akkawi R, et al. Cell Death Dis. 2024 Jan 5;15(1):13. doi: 10.1038/s41419-023-06378-8. Cell Death Dis. 2024. PMID: 38182577 Free PMC article. - mTORC1 Signaling Promotes Osteoblast Differentiation from Preosteoblasts.
Chen J, Long F. Chen J, et al. PLoS One. 2015 Jun 19;10(6):e0130627. doi: 10.1371/journal.pone.0130627. eCollection 2015. PLoS One. 2015. PMID: 26090674 Free PMC article. - Multiparametric senescent cell phenotyping reveals targets of senolytic therapy in the aged murine skeleton.
Doolittle ML, Saul D, Kaur J, Rowsey JL, Vos SJ, Pavelko KD, Farr JN, Monroe DG, Khosla S. Doolittle ML, et al. Nat Commun. 2023 Jul 31;14(1):4587. doi: 10.1038/s41467-023-40393-9. Nat Commun. 2023. PMID: 37524694 Free PMC article.
References
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, et al. (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29. - PubMed
- Long F (2012) Building strong bones: molecular regulation of the osteoblast lineage. Nature reviews Molecular cell biology 13: 27–38. - PubMed
- Hilton MJ, Tu X, Cook J, Hu H, Long F (2005) Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development 132: 4339–4351. - PubMed
- Long F (2011) Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 13: 27–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR055923/AR/NIAMS NIH HHS/United States
- DK065789/DK/NIDDK NIH HHS/United States
- R01 DK065789/DK/NIDDK NIH HHS/United States
- R01 HD049808/HD/NICHD NIH HHS/United States
- R01 AR055923/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases