Sorting out the trash: the spatial nature of eukaryotic protein quality control - PubMed (original) (raw)
Review
Sorting out the trash: the spatial nature of eukaryotic protein quality control
Emily Mitchell Sontag et al. Curr Opin Cell Biol. 2014 Feb.
Abstract
Failure to maintain protein homeostasis is associated with aggregation and cell death, and underies a growing list of pathologies including neurodegenerative diseases, aging, and cancer. Misfolded proteins can be toxic and interfere with normal cellular functions, particularly during proteotoxic stress. Accordingly, molecular chaperones, the ubiquitin-proteasome system (UPS) and autophagy together promote refolding or clearance of misfolded proteins. Here we discuss emerging evidence that the pathways of protein quality control (PQC) are intimately linked to cell architecture, and sequester proteins into spatially and functionally distinct PQC compartments. This sequestration serves a number of functions, including enhancing the efficiency of quality control; clearing the cellular milieu of potentially toxic species and facilitating asymmetric inheritance of damaged proteins to promote rejuvenation of daughter cells.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
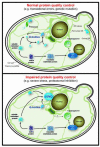
Figure 1. The spatial distribution of the different PQC compartments within a yeast cell
Model illustrating the different PQC compartments and their subcellular locations in yeast grown under normal growth conditions (top) or conditions of impaired protein quality control (bottom). Similar compartments have been described in mammalian cells, including the aggresome and ALIS (see text for details).
Figure 2. Pathways of spatial processing of misfolded proteins within the cell
Stress-denatured proteins interact with a number of sorting factors directing the misfolded proteins to individual PQC compartments within the cell, leading to refolding, degradation or terminal sequestration of the potentially toxic species.
Similar articles
- Protein Quality Control by Molecular Chaperones in Neurodegeneration.
Ciechanover A, Kwon YT. Ciechanover A, et al. Front Neurosci. 2017 Apr 6;11:185. doi: 10.3389/fnins.2017.00185. eCollection 2017. Front Neurosci. 2017. PMID: 28428740 Free PMC article. Review. - Spatially organized aggregation of misfolded proteins as cellular stress defense strategy.
Miller SB, Mogk A, Bukau B. Miller SB, et al. J Mol Biol. 2015 Apr 10;427(7):1564-74. doi: 10.1016/j.jmb.2015.02.006. Epub 2015 Feb 11. J Mol Biol. 2015. PMID: 25681695 Review. - Spatial sequestration of misfolded proteins in neurodegenerative diseases.
Rolli S, Sontag EM. Rolli S, et al. Biochem Soc Trans. 2022 Apr 29;50(2):759-771. doi: 10.1042/BST20210862. Biochem Soc Trans. 2022. PMID: 35311889 Review. - Molecular chaperones and proteostasis regulation during redox imbalance.
Niforou K, Cheimonidou C, Trougakos IP. Niforou K, et al. Redox Biol. 2014 Jan 30;2:323-32. doi: 10.1016/j.redox.2014.01.017. eCollection 2014. Redox Biol. 2014. PMID: 24563850 Free PMC article. Review. - Distinct proteostasis circuits cooperate in nuclear and cytoplasmic protein quality control.
Samant RS, Livingston CM, Sontag EM, Frydman J. Samant RS, et al. Nature. 2018 Nov;563(7731):407-411. doi: 10.1038/s41586-018-0678-x. Epub 2018 Oct 31. Nature. 2018. PMID: 30429547 Free PMC article.
Cited by
- Endoplasmic reticulum network heterogeneity guides diffusive transport and kinetics.
Scott ZC, Koning K, Vanderwerp M, Cohen L, Westrate LM, Koslover EF. Scott ZC, et al. Biophys J. 2023 Aug 8;122(15):3191-3205. doi: 10.1016/j.bpj.2023.06.022. Epub 2023 Jul 3. Biophys J. 2023. PMID: 37401053 Free PMC article. - Targeting Autophagy in Atrial Fibrillation.
Ye Q, Chen W, Fu H, Ding Y, Jing Y, Shen J, Yuan Z, Zha K. Ye Q, et al. Rev Cardiovasc Med. 2023 Oct 8;24(10):288. doi: 10.31083/j.rcm2410288. eCollection 2023 Oct. Rev Cardiovasc Med. 2023. PMID: 39077569 Free PMC article. Review. - Proteotoxic stress promotes entrapment of ribosomes and misfolded proteins in a shared cytosolic compartment.
Ghosh A, Williams LD, Pestov DG, Shcherbik N. Ghosh A, et al. Nucleic Acids Res. 2020 Apr 17;48(7):3888-3905. doi: 10.1093/nar/gkaa068. Nucleic Acids Res. 2020. PMID: 32030400 Free PMC article. - Prion aggregate structure in yeast cells is determined by the Hsp104-Hsp110 disaggregase machinery.
O'Driscoll J, Clare D, Saibil H. O'Driscoll J, et al. J Cell Biol. 2015 Oct 12;211(1):145-58. doi: 10.1083/jcb.201505104. Epub 2015 Oct 5. J Cell Biol. 2015. PMID: 26438827 Free PMC article. - The biology of proteostasis in aging and disease.
Labbadia J, Morimoto RI. Labbadia J, et al. Annu Rev Biochem. 2015;84:435-64. doi: 10.1146/annurev-biochem-060614-033955. Epub 2015 Mar 12. Annu Rev Biochem. 2015. PMID: 25784053 Free PMC article. Review.
References
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. - PubMed
- Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2010;40:238–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32NS086253-01/NS/NINDS NIH HHS/United States
- R01 GM056433/GM/NIGMS NIH HHS/United States
- R01 GM074074/GM/NIGMS NIH HHS/United States
- PN2 EY016525/EY/NEI NIH HHS/United States
- F32 NS086253/NS/NINDS NIH HHS/United States
- R37 GM056433/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources