Untangling the influences of voluntary running, environmental complexity, social housing and stress on adult hippocampal neurogenesis - PubMed (original) (raw)
Untangling the influences of voluntary running, environmental complexity, social housing and stress on adult hippocampal neurogenesis
Catherine-Alexandra Grégoire et al. PLoS One. 2014.
Abstract
Environmental enrichment (EE) exerts powerful effects on brain physiology, and is widely used as an experimental and therapeutic tool. Typical EE paradigms are multifactorial, incorporating elements of physical exercise, environmental complexity, social interactions and stress, however the specific contributions of these variables have not been separable using conventional housing paradigms. Here, we evaluated the impacts of these individual variables on adult hippocampal neurogenesis by using a novel "Alternating EE" paradigm. For 4 weeks, adult male CD1 mice were alternated daily between two enriched environments; by comparing groups that differed in one of their two environments, the individual and combinatorial effects of EE variables could be resolved. The Alternating EE paradigm revealed that (1) voluntary running for 3 days/week was sufficient to increase both mitotic and post-mitotic stages of hippocampal neurogenesis, confirming the central importance of exercise; (2) a complex environment (comprised of both social interactions and rotated inanimate objects) had no effect on neurogenesis itself, but enhanced depolarization-induced c-Fos expression (attributable to social interactions) and buffered stress-induced plasma corticosterone levels (attributable to inanimate objects); and (3) neither social isolation, group housing, nor chronically increased levels of plasma corticosterone had a prolonged impact on neurogenesis. Mouse strain, handling and type of running apparatus were tested and excluded as potential confounding factors. These findings provide valuable insights into the relative effects of key EE variables on adult neurogenesis, and this "Alternating EE" paradigm represents a useful tool for exploring the contributions of individual EE variables to mechanisms of neural plasticity.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
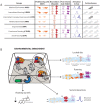
Figure 1. The Alternating EE paradigm.
A, Timeline of the Alternating EE paradigm. Each experimental group was alternated between two types of housing environments 6 times/week for a period of 4 weeks. Each mouse received two injections of BrdU on the first day of the paradigm. B, Basic environments used in the Alternating EE paradigm: Empty (devoid of all items, in which mice are either isolated or socially housed in groups of 3), Locked disc (containing a running disc that has been locked to prevent running exercise), Running disc (containing a normal running disc), and Complex (a Locked disc cage to which multi-colored tunnels have been added (rotated 4 times/week) and 3 mice are socially housed) (see Methods for additional details). C. Alternating EE experimental groups: Each experimental group was built using 2 of the basic environments in B. Mice in each group alternated daily between these two basic environments for the entire 4 week period. D, Statistical contrasts: For each parameter measured in this study, a difference detected by one-way ANOVA was followed by the testing of 7 specific and pre-determined hypotheses. Each of these 7 statistical contrasts is made between two experimental groups that differ in only one EE variable. No statistical analyses were made between groups in which more than one variable differed.
Figure 2. Effects of Alternating EE on the main stages of dentate gyrus neurogenesis.
A–D, Quantification of the density of A, Ki67+ proliferating cells, B, NeuroD+ neuroblasts, C, Calretinin+ maturing post-mitotic neurons, and D, BrdU+ surviving cells. At the right of each panel is a sample cresyl-violet counterstaining (A–C) or NeuN-labeling (D) (upper left image), and representative non-counterstained sections from MIN (upper right), I-RUN (lower left) and I-CPX (lower right) experimental groups. Note that a main significant effect of the Running disc environment (I-RUN) was detected for all four neurogenesis markers, while the Complex environment (I-CPX) did not have a main effect on any marker and did not potentiate the effects of running. In D, middle panel, quantification of the percentage of BrdU+ cells that co-express NeuN, DCX or S100β. See Results for further details. Scale bar = 50 µm. DG = Dentate Gyri.
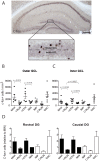
Figure 3. Effects of Alternating EE on depolarization-associated c-fos expression.
A, Low magnification image of c-fos immunohistochemistry on a coronal section of the hippocampus. Dotted line in the enlarged box illustrates how the granule cell layer (GCL) was divided into outer GCL and inner GCL for quantification purposes. B,C Quantifications of c-fos expression in the B, Outer GCL and C, Inner GCL. Note that a positive effect of the Complex environment (I-CPX) was detectable in both the Inner GCL and Outer GCL, while the Running disc environment (I-RUN) had a negative effect in the Inner GCL only. Socially-housed mice in empty cages (SOC) also exhibited increased c-fos expression compared to isolated mice (IMP). See Results for further details. D, Rostral-caudal distribution of c-fos-expressing cells in the DG. The rostral and caudal tissue sections correspond to the dorsal and ventral hippocampus regions, respectively. Note that the pattern of c-fos expression across groups is virtually identical in both the rostral and caudal DG. Scale bar: 250 µm. DG = Dentate Gyri. GCL = Granule Cell Layer.
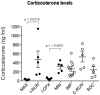
Figure 4. Plasma corticosterone concentrations are reduced in the Complex environment.
Plasma corticosterone concentration was measured by ELISA. Note that corticosterone levels are high in all experimental groups except those that include the Complex environment (I-CPX, MAX) See Results for further details.
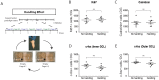
Figure 5. Daily handling does not affect basal neurogenesis in the Alternating EE paradigm.
A, Timeline of the Handling Effect experiment. Mice were either housed as for the IMP group of the Intermittent EE paradigm (i.e., Empty environment with daily handling) or in an Empty housing condition without daily handling for 4 weeks. B–E, Quantifications of the number of B, Ki67+ proliferating cells, C, Calretinin+ maturing post-mitotic neurons, D, Inner GCL c-fos+ cells or E, outer GCL c-fos+ cells. No significant effects of daily handling on these markers were detected (t-tests). See Results for statistical details. DG = Dentate Gyri.
Figure 6. Comparison of the effects of running wheels and running discs on adult neurogenesis.
A, The Wheel versus Disc experiment. Mice were maintained for 4 weeks in one of five housing conditions: Empty, Locked wheel, Running wheel, Locked disc and Running disc. B, Comparison of average daily running distances on Running Wheels and Running Discs. Note that average running distances were about 80% higher on Discs than on Wheels. C–E, Quantifications of the numbers of C, Ki67+ proliferating cells, D, NeuroD+ neuroblasts and E, Calretinin+ maturing post-mitotic neurons. In all cases, there was no significant difference between the Control, Locked wheel and Locked disc groups, or between the Running wheel and Running disc groups (t-tests). DG = Dentate Gyri.
Figure 7. Summary of long-term effects of individual EE variables on hippocampal neurogenesis, c-fos expression and corticosterone levels.
A, Table summarizing relative changes to DG neurogenesis, depolarization-associated c-fos expression within the granule cell layer, and plasma corticosterone levels in the 4-week Alternating EE paradigm. Changes relative to the MIN group are highlighted. B, The EE variables examined in the present study included running, environmental complexity, social context (isolation and social enrichment) and stress-associated plasma corticosterone. Voluntary running strongly increased all stages of neurogenesis compared to exposure to a locked disc. Continuous voluntary running did not have a greater neurogenic effect than intermittent running. Environmental complexity (involving a combination of inanimate objects, social interactions and conformational novelty) did not affect basal or running-induced neurogenesis, but enhanced depolarization-associated c-fos expression within the granule cell layer (likely due to Social interactions) and decreased plasma corticosterone concentrations (likely due to physical Complexity associated with tunnels and toys). Chronic differences in the levels of stress-associated corticosterone had no detectable positive or negative effects on running-induced neurogenesis and were not responsible for preventing effects of environmental complexity on neurogenesis. The baseline environment, a Locked disc, had no detectable impact on any stage of neurogenesis, neuronal activation or corticosterone levels when compared to a completely impoverished environment. Thus, the Alternating EE paradigm enables dissociation of the impacts of distinct elements of environmental enrichment.
Similar articles
- Protective neuroendocrine effects of environmental enrichment and voluntary exercise against social isolation: evidence for mediation by limbic structures.
Watanasriyakul WT, Normann MC, Akinbo OI, Colburn W, Dagner A, Grippo AJ. Watanasriyakul WT, et al. Stress. 2019 Sep;22(5):603-618. doi: 10.1080/10253890.2019.1617691. Epub 2019 May 28. Stress. 2019. PMID: 31134849 Free PMC article. - Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice.
Kannangara TS, Webber A, Gil-Mohapel J, Christie BR. Kannangara TS, et al. Hippocampus. 2009 Oct;19(10):889-97. doi: 10.1002/hipo.20514. Hippocampus. 2009. PMID: 18958850 - The neurogenic effects of an enriched environment and its protection against the behavioral consequences of chronic mild stress persistent after enrichment cessation in six-month-old female Balb/C mice.
Vega-Rivera NM, Ortiz-López L, Gómez-Sánchez A, Oikawa-Sala J, Estrada-Camarena EM, Ramírez-Rodríguez GB. Vega-Rivera NM, et al. Behav Brain Res. 2016 Mar 15;301:72-83. doi: 10.1016/j.bbr.2015.12.028. Epub 2015 Dec 22. Behav Brain Res. 2016. PMID: 26721469 - Differential impact of stress and environmental enrichment on corticolimbic circuits.
Smail MA, Smith BL, Nawreen N, Herman JP. Smail MA, et al. Pharmacol Biochem Behav. 2020 Oct;197:172993. doi: 10.1016/j.pbb.2020.172993. Epub 2020 Jul 11. Pharmacol Biochem Behav. 2020. PMID: 32659243 Free PMC article. Review. - Social behavior, hormones and adult neurogenesis.
Opendak M, Briones BA, Gould E. Opendak M, et al. Front Neuroendocrinol. 2016 Apr;41:71-86. doi: 10.1016/j.yfrne.2016.02.002. Epub 2016 Mar 17. Front Neuroendocrinol. 2016. PMID: 26996817 Review.
Cited by
- Dissociating the therapeutic effects of environmental enrichment and exercise in a mouse model of anxiety with cognitive impairment.
Rogers J, Vo U, Buret LS, Pang TY, Meiklejohn H, Zeleznikow-Johnston A, Churilov L, van den Buuse M, Hannan AJ, Renoir T. Rogers J, et al. Transl Psychiatry. 2016 Apr 26;6(4):e794. doi: 10.1038/tp.2016.52. Transl Psychiatry. 2016. PMID: 27115125 Free PMC article. - Structural and Functional Hippocampal Correlations in Environmental Enrichment During the Adolescent to Adulthood Transition in Mice.
Manno FAM, Kumar R, An Z, Khan MS, Su J, Liu J, Wu EX, He J, Feng Y, Lau C. Manno FAM, et al. Front Syst Neurosci. 2022 Feb 15;15:807297. doi: 10.3389/fnsys.2021.807297. eCollection 2021. Front Syst Neurosci. 2022. PMID: 35242015 Free PMC article. - Mechanisms underlying the effect of voluntary running on adult hippocampal neurogenesis.
Gao Y, Syed M, Zhao X. Gao Y, et al. Hippocampus. 2023 Apr;33(4):373-390. doi: 10.1002/hipo.23520. Epub 2023 Mar 9. Hippocampus. 2023. PMID: 36892196 Free PMC article. Review. - Stearoyl-CoA Desaturase inhibition reverses immune, synaptic and cognitive impairments in an Alzheimer's disease mouse model.
Hamilton LK, Moquin-Beaudry G, Mangahas CL, Pratesi F, Aubin M, Aumont A, Joppé SE, Légiot A, Vachon A, Plourde M, Mounier C, Tétreault M, Fernandes KJL. Hamilton LK, et al. Nat Commun. 2022 Apr 20;13(1):2061. doi: 10.1038/s41467-022-29506-y. Nat Commun. 2022. PMID: 35443751 Free PMC article. - Evolutionary Basis of Human Running and Its Impact on Neural Function.
Schulkin J. Schulkin J. Front Syst Neurosci. 2016 Jul 11;10:59. doi: 10.3389/fnsys.2016.00059. eCollection 2016. Front Syst Neurosci. 2016. PMID: 27462208 Free PMC article. Review.
References
- Wiesel TN, Hubel DH (1965) Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol 28: 1060–1072. - PubMed
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC (1962) Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J Comp Physiol Psychol 55: 429–437. - PubMed
- Hebb DO (1947) The effects of early experience on problem solving at maturity. American Psychologist 2: 306–307.
- La Torre JC (1968) Effect of differential environmental enrichment on brain weight and on acetylcholinesterase and cholinesterase activities in mice. Exp Neurol 22: 493–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical