MiR-26a promotes ovarian cancer proliferation and tumorigenesis - PubMed (original) (raw)
MiR-26a promotes ovarian cancer proliferation and tumorigenesis
Wenjing Shen et al. PLoS One. 2014.
Abstract
MicroRNAs (miRNAs) important for posttranscriptional gene expression are involved in the initiation and progression of human cancer. In this study, we reported that miR-26a was over-expressed in human EOC specimens and the expression level of extracellular miR-26a in plasma can distinguish patients from healthy controls in EOC. Ectopic expression of miR-26a in ovarian cancer (OC) cells increased cell proliferation and clonal formation. This growth promoting effect of OC cell growth was mediated by miR-26a inhibition of the posttranscription of ER-α. Furthermore, inhibition of miR-26a suppressed the tumor formation generated by injecting OC cells in nude mice. Our results suggest that aberrantly expressed miR-26a may contribute to OC development.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
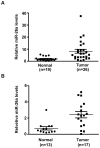
Figure 1. The expression of miR-26a was increased in EOC patients.
(A)Quantitative analysis of the expression levels of miR-26a in EOC samples normalized to those of 18s rRNA by qRT-PCR. Data for each dot were mean value of one sample repeated in three independent experiments (normal, n = 19; tumor, n = 26). **P<0.01 vs Normal. (B)Quantitative analysis of the expression levels of plasma miR-26a by qRT-PCR. Data for each dot were mean value of one sample repeated in three independent experiments (normal, n = 13; tumor, n = 17).
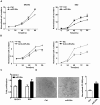
Figure 2. MiR-26a promoted EOC cell growth.
Growth curves of SKOV3 (A) or ES2 (B) cells transfected with miR-26a (left panel) or anti-miR-26a (right panel). Cell numbers were normalized to those in 0 hr. Data were mean±s.e. of three independent experiments in triplicate. *p<0.05, **p<0.01 vs empty vector (Ctrl) or negative control (NC). (C) Cell proliferation transfected with miR-26a was measured by a WST-1 assay. Data were mean±s.e. of three independent experiments in triplicate. **p<0.01 vs empty vector (Ctrl) (D) Quantification of the colonies formed by Ctrl or miR-26a transfected cells. Data were mean±s.e. of three independent experiments. **P<0.01 vs Ctrl.
Figure 3. ERα was a target of miR-26a.
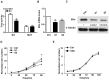
(A) Stable 1 cells (S1) were transfected with pGL3-ERα-WT (WT) reporter vector or pGL3-ERα-Mut (MUT). Data were mean±s.e. of three independent experiments. **P<0.01 vs control cells (Ctrl). (B) Quantitative analysis of the expression levels of ERα in Stable1 (S1), Stable2 (S2) and control (Ctrl) cells were determined and normalized to GAPDH mRNA levels. *p<0.05,**P<0.01 vs Ctrl. (C) Western-blot analysis of total cell lysates extracted from indicated stable cells using the indicated antibodies. Data was representative of three independent experiments and quantitation normalized with the Ctrl. Tubulin served as a loading control. (D) The growth curves of S1, S2 and Ctrl cells. Data were mean±s.e. of three independent experiments in triplicate. *p<0.05, **P<0.01 vs Ctrl. (E) Over-expression of ERα decreased the growth of S1 cells after transfection. Cell numbers were normalized to Ctrl cells. Data were mean±s.e. of three independent experiments in triplicate.
Figure 4. MiR-26a promoted the development of tumor in nude mice.
Tumor formation generated by the SKOV3 cells. (A) transfected with miR-26a or anti-miR-26a(B) Nude mice were subcutaneously injected with 2×106 transfected cells. Representative images, measurement of the final volume and weight of the tumors formed. The tumor sizes were measured and calculated in Materials and methods. Data were mean±s.d. of 4–6 mice. **P<0.01 vs empty vector or nonsense.
Similar articles
- MiR-125b targets BCL3 and suppresses ovarian cancer proliferation.
Guan Y, Yao H, Zheng Z, Qiu G, Sun K. Guan Y, et al. Int J Cancer. 2011 May 15;128(10):2274-83. doi: 10.1002/ijc.25575. Int J Cancer. 2011. PMID: 20658525 - Inhibition of Ovarian Epithelial Carcinoma Tumorigenesis and Progression by microRNA 106b Mediated through the RhoC Pathway.
Chen S, Chen X, Xiu YL, Sun KX, Zhao Y. Chen S, et al. PLoS One. 2015 May 1;10(5):e0125714. doi: 10.1371/journal.pone.0125714. eCollection 2015. PLoS One. 2015. PMID: 25933027 Free PMC article. - MicroRNA-26a inhibits angiogenesis by down-regulating VEGFA through the PIK3C2α/Akt/HIF-1α pathway in hepatocellular carcinoma.
Chai ZT, Kong J, Zhu XD, Zhang YY, Lu L, Zhou JM, Wang LR, Zhang KZ, Zhang QB, Ao JY, Wang M, Wu WZ, Wang L, Tang ZY, Sun HC. Chai ZT, et al. PLoS One. 2013 Oct 23;8(10):e77957. doi: 10.1371/journal.pone.0077957. eCollection 2013. PLoS One. 2013. PMID: 24194905 Free PMC article. - MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2.
Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, Chen YC, Peng Y, Yao KT, Kung HF, Li XP. Lu J, et al. Cancer Res. 2011 Jan 1;71(1):225-33. doi: 10.1158/0008-5472.CAN-10-1850. Cancer Res. 2011. PMID: 21199804 - The loss of miR-26a-mediated post-transcriptional regulation of cyclin E2 in pancreatic cancer cell proliferation and decreased patient survival.
Deng J, He M, Chen L, Chen C, Zheng J, Cai Z. Deng J, et al. PLoS One. 2013 Oct 8;8(10):e76450. doi: 10.1371/journal.pone.0076450. eCollection 2013. PLoS One. 2013. PMID: 24116110 Free PMC article.
Cited by
- An overview of long non-coding RNAs in ovarian cancers.
Meryet-Figuière M, Lambert B, Gauduchon P, Vigneron N, Brotin E, Poulain L, Denoyelle C. Meryet-Figuière M, et al. Oncotarget. 2016 Jul 12;7(28):44719-44734. doi: 10.18632/oncotarget.8089. Oncotarget. 2016. PMID: 26992233 Free PMC article. Review. - Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer.
Sassu CM, Palaia I, Boccia SM, Caruso G, Perniola G, Tomao F, Di Donato V, Musella A, Muzii L. Sassu CM, et al. Int J Mol Sci. 2021 Dec 20;22(24):13650. doi: 10.3390/ijms222413650. Int J Mol Sci. 2021. PMID: 34948446 Free PMC article. Review. - Characterization of microRNA expression in B cells derived from Japanese black cattle naturally infected with bovine leukemia virus by deep sequencing.
Ochiai C, Miyauchi S, Kudo Y, Naruke Y, Yoneyama S, Tomita K, Dongze L, Chiba Y, Hirata TI, Ichijo T, Nagai K, Kobayashi S, Yamada S, Hikono H, Murakami K. Ochiai C, et al. PLoS One. 2021 Sep 10;16(9):e0256588. doi: 10.1371/journal.pone.0256588. eCollection 2021. PLoS One. 2021. PMID: 34506539 Free PMC article. - Plasma miR-454-3p as a potential prognostic indicator in human glioma.
Shao N, Wang L, Xue L, Wang R, Lan Q. Shao N, et al. Neurol Sci. 2015 Feb;36(2):309-13. doi: 10.1007/s10072-014-1938-7. Epub 2014 Sep 6. Neurol Sci. 2015. PMID: 25190548 - Loss of miR-26a-5p promotes proliferation, migration, and invasion in prostate cancer through negatively regulating SERBP1.
Guo K, Zheng S, Xu Y, Xu A, Chen B, Wen Y. Guo K, et al. Tumour Biol. 2016 Sep;37(9):12843-12854. doi: 10.1007/s13277-016-5158-z. Epub 2016 Jul 23. Tumour Biol. 2016. PMID: 27449037
References
- Hoskins WJ (1995) Prospective on ovarian cancer: why prevent? J Cell Biochem Suppl 23: 189–199. - PubMed
- Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9: 102–114. - PubMed
- Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6: 857–866. - PubMed
- Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, et al. (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64: 3753–3756. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical