High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury - PubMed (original) (raw)
High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury
Teresa A Evans et al. Exp Neurol. 2014 Apr.
Abstract
After traumatic spinal cord injury, functional deficits increase as axons die back from the center of the lesion and the glial scar forms. Axonal dieback occurs in two phases: an initial axon intrinsic stage that occurs over the first several hours and a secondary phase which takes place over the first few weeks after injury. Here, we examine the secondary phase, which is marked by infiltration of macrophages. Using powerful time-lapse multi-photon imaging, we captured images of interactions between Cx3cr1(+/GFP) macrophages and microglia and Thy-1(YFP) axons in a mouse dorsal column crush spinal cord injury model. Over the first few weeks after injury, axonal retraction bulbs within the lesion are static except when axonal fragments are lost by a blebbing mechanism in response to physical contact followed by phagocytosis by mobile Cx3Cr1(+/GFP) cells. Utilizing a radiation chimera model to distinguish marrow-derived cells from radio-resistant CNS-resident microglia, we determined that the vast majority of accumulated cells in the lesion are derived from the blood and only these are associated with axonal damage. Interestingly, CNS-resident Cx3Cr1(+/GFP) microglia did not increasingly accumulate nor participate in neuronal destruction in the lesion during this time period. Additionally, we found that the blood-derived cells consisted mainly of singly labeled Ccr2(+/RFP) macrophages, singly labeled Cx3Cr1(+/GFP) macrophages and a small population of double-labeled cells. Since all axon destructive events were seen in contact with a Cx3Cr1(+/GFP) cell, we infer that the CCR2 single positive subset is likely not robustly involved in axonal dieback. Finally, in our model, deletion of CCR2, a chemokine receptor, did not alter the position of axons after dieback. Understanding the in vivo cellular interactions involved in secondary axonal injury may lead to clinical treatment candidates involving modulation of destructive infiltrating blood monocytes.
Keywords: Axonal dieback; Bone marrow chimera mice; CCR2; CX3CR1; Macrophages; Microglia; Monocytes; Retraction bulb; Spinal cord injury; Two-photon microscopy.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
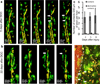
Figure 1. Axons die back from the site of injury and macrophages infiltrate into a physiologic lesion of the dorsal columns in double transgenic Thy-1 and CX3CR1 GFP/+ mice
a) Diagram of the DCC injury. The red portion of the cord is damaged by a pinching motion of forceps. b) The DCC lesion was easily measured in double transgenic Cx3cr1+/GFP (green) × Thy-1+/YFP (yellow) animals with blood vessels labeled by intravenous TRITC-dextran vessel dye injection (red). The lesion is located by the insertion points of the two forceps tines (dotted pink lines). A line is then drawn between the centers of each insertion site to locate the center of the lesion (dotted orange line). Axon position is measured as the distance between axon tips and the orange line (white lines). Scale bar = 200 um. c) Imaging of the DCC lesion in four separate animals before injury and during the first 8 days post injury reveals intact axons (yellow) in the dorsal column before injury and axonal retraction bulbs at progressively longer distances from the center of the lesion over time. After injury, the axonal position at the edge of the injury site is marked with a white dotted line. As time after injury increases, numbers of Cx3cr1+/GFP cells (green) within both the lesion border and the center of the lesion increase. Scale bar = 100 um. d) Higher magnification of the end bulbs after injury, matching closely to those shown by Cajal (Cajal, 1907). e) Measurement of axon distances from center of DCC lesion. Axons die back an average of 336 µm over the first 8 days after injury. N=3 at 0 and 2 days, N=4 at 5 and 8 days. * P<0.05, ** P<0.01, ***P<0.001. f) Quantification of Cx3cr1+/GFP cell infiltration into center of DC lesion as in C. The number of CX3CR1_+/GFP_ macrophages and microglia increases steadily over the first 8 days. N=3 at 0 and 2 days, N=4 at 5 and 8 days * P=<0.05 ** P<0.0001, ***P<0.0002 g) Example of flow cytometry analysis of dissociated spinal cord 5 days after injury showing increased number of double positive, CD11b+ × Cx3cr1+/GFP cells found in the injured spinal cord, but not in an animal with a sham laminectomy. h) Flow cytometry analyses from blood, spleen and dissociated spine of Cx3cr1+/GFP mice 5 days after DCC injury compared to animals receiving a laminectomy but no injury. For both g and h, sample was gated to include all live cells in the sample of dissociated spinal cord. No additional purification steps were taken. Double positive Cx3cr1+/GFP / CD11b+ macrophage numbers remain the same in the blood and spleen but increased significantly in the spine 5 days after injury. Each condition was run in triplicate. * P<0.05. All error bars are the standard error of the mean. P values are derived using a two-tailed t-test for two samples of unequal variance.
Figure 2. Cx3cr1+/GFP cells contact axons in the dorsal column after initial DCC injury at sites of axonal thinning and breaking
Examples of axons (yellow) interacting with Cx3cr1+/GFP cells (green) at different times following DCC injury. Yellow axons are ascending dorsal column axons approaching the lesion. The lesion center is above the area shown. a) Example of interaction 5 days after DCC injury. Thinning and blebbing of the axon are marked by white arrows. Time stamp = hrs:mins, total time 2 hours. Scale bar =20 um. b) Example of an axonal cleavage 22 days after DCC injury. Cellular material can be seen separating from the end of the dystrophic axon after contact by a Cx3cr1+/GFP cell (white arrow). This indicates that loss of axonal bits is a continuing process even at later time points after injury. Time stamp = hrs:mins. Total time: 1 hour and 30 minutes. Scale bar =20 um. c) Quantification of the rate of Cx3cr1+/GFP cell contact with axons. Contacts were registered when a green Cx3cr1+/GFP cell remained less than 100um from an axon for more than 2 minutes. A destructive contact was registered when the width of the axon changed by more than 50% at a location with Cx3cr1+/GFP cell contact. Once a contact between a Cx3cr1+/GFP cell was identified, the width of the axon at the time of contact was measured in Imaris and compared to the width of the axon at the end of the imaging session or point where the contact ended, whichever came first. Non-destructive contacts (hashed bars) occurred more frequently than destructive ones, and increased on day 8 after DCC injury. Destructive contacts (black bars) were present in small numbers at 2,5 and 8 days after injury. We observed a total of 45 contacts and 2 destructive contacts in 1.3 hours of imaging in 3 animals at 2 days after injury, a total of 162 contacts and 6 destructive contacts in 4.65 hours of imaging in 5 animals at 5 days after injury and total of 98 contacts and 3 destructive contacts in 5.4 hours of imaging in 4 animals at 8 days after injury. Error bars are the standard error of the mean. d) Image of the lesion near axonal retraction bulbs at 5 days after injury. Axons (yellow), Cx3cr1+/GFP cells (green) and the dorsal vein (V, labeled with TRITC dextran, red) can be seen. Yellow bits of axon material (arrows) can be seen in the lesion along the path that axons would have retracted along. These yellow inclusions are commonly seen along the edges of the lesion near retracting axons and appear to be parts of axons left behind during the process of axonal retraction. Scale bar = 50 um.
Figure 3. CX3CR1+ cells phagocytize the ends of damaged dorsal column axons attached to the neuronal cell bodies in the dorsal root ganglia
a) Experimental scheme is depicted. PH-sensitive PhRodo-conjugated dextran (10,000 MW) was injected into the sciatic nerve unilaterally on day 6 following DCC injury. The dye was then taken up in the crushed sciatic nerve neurons and carried rostrally inside the neuron through the dorsal column to the site of DCC injury (yellow). The dye fluoresces red after phagocytosis and inclusion in a low pH lysosome. This allows us to distinguish axonal debris take up by macrophages that is removed from the ends of axons between days 6 and 8 from debris from the initial injury or from distal axons undergoing Wallerian degeneration. b) The PhRodo dye was seen inside Cx3cr1+/GFP phagocytic cells located within DCC lesion ipsilaterally to the sciatic injection. The average number of puncta on the side of injection was 43±2, compared to 17±10 puncta on the contralateral spine. c) Reconstruction of a Cx3cr1+/GFP cell seen in Figure 3B with PhRodo dye inclusions, showing that these inclusions are within the green Cx3cr1+/GFP cells. d) Quantification of PhRodo inclusions in vesicles on the contralateral and ipsilateral side of the animal to dye injection. We observed a total of 53 inclusions on the ipsilateral side to injection and 25 inclusions on the contralateral side in a total of 3 Cx3cr1+/GFP mice. Error bars are standard error of the mean. *P<0.05 using a two-tailed t-test assuming two samples of unequal variance. E) In the absence of PhRodo injection and sciatic nerve manipulation and any corresponding conditioning effects, Thy-1YFP inclusions (white) can also be found within Cx3cr1+/GFP cells (green) near dystrophic axon ends (white) 5 days following DCC injury without manipulation of the sciatic nerve. CX3CR1+ cells were found along the ends of dystrophic axons. f) A single Cx3cr1+/GFP cell (green) with Thy-1YFP inclusions (white) shown in E. X, Y and Z projections are shown, as well as a reconstruction of this cell showing the white inclusions within the Cx3cr1+/GFP cell.
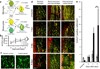
Figure 4. The bulk of CX3CR1+ cells within DCC lesion are blood-derived
a) A schematic diagram of the bone marrow chimeric mice approach to allow for the distinction of blood-derived monocyte-lineage macrophages from resident microglia Cx3cr1+/GFP cells. Recipient mice were irradiated, reconstituted with donor-derived bone marrow cells and then allowed to rest for 8–12 weeks before imaging. Cx3cr1+/GFP × _Thy-1YFP/+_animals received wild type B6 bone marrow, and Thy-1YFP/+ animals received CX3CR1+ bone marrow, allowing only microglia or only blood derived cells to be CX3CR1+. b) Quantification of post-DCC axonal dieback in chimeric animals 8–12 weeks after transplant revealed no significant rate difference. All conditions have Ns of between 2 and 7. The average N is 4. A total of 639 axons in 98 mice were imaged. c) Comparison of microglia in chimeric animals with a Cx3cr1+/GFP host and a B6 donor and Cx3cr1+/GFP animals shows similar morphology. Scale bar is 50 um. Non-significance was determined using a two-tailed t-test assuming two sample of unequal variance. d) Images of the caudal aspect of the DCC lesion in Thy-1 YFP/+ chimeric animals with either labeled host or donor Cx3cr1+/GFP cells as compared to non-chimeric, un-irradiated double transgenic Thy-1YFP × Cx3cr1+/GFP animals. The first column shows animals with Cx3cr1+/GFP hosts and B6 donors, showing resident microglia. Small numbers of Cx3cr1+/GFP positive cells are present in the lesion at all time points. The center column shows animals with Cx3cr1+/GFP donor cells, showing blood derived Cx3cr1+/GFP monocytes and macrophages that rapidly increase in number with increasing time after injury. The right column shows non-chimeric double transgenic Thy-1YFP × Cx3cr1+/GFP animals. e) Quantification of cell numbers in the center of the lesion in E revealed consistently few microglia (hashed bars), but a dramatic increase in monocyte-derived cell number (black bars) over 8 days. Microglia numbers remain stable over time, and infiltrating macrophages increase dramatically over time. All conditions have Ns of between 2 and 7. The average N is 4. Number of microglia and macrophages at the same time point are statistically different at days 2 and 8 after injury. *P<0.02 using a one tailed T-test assuming two samples of unequal variance.
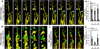
Figure 5. Blood-derived CX3CR1+ cells have greater average speed and displacement than resident microglia
a) Immediately after DCC lesion, Cx3cr1+/GFP microglia move only very short distances, while blood-derived cells traversed over great distances within the center of the lesion. Cell tracks are shown as color-coded time stamps (blue: Time = 0 min; yellow: time = 60 mins; gradient shown in figure). Both tracks and fluorescence are shown in images on the left, and only tracks in images on the right. Scale bar = 50um. Total time for tracking is one hour. b) High-magnification sequential images of a one macrophage’s track and movement in a. Scale bar = 7 um. Time stamp: hrs:mins. Total time shown is 28 minutes. c) 8 days after DCC, only a few stationary microglial cells were found within the lesion 8 days following DCC injury. In comparison, blood-derived cells traveled over greater distances near the lesion. Both tracks and fluorescence are shown in images on the left, and only tracks in images on the right. Time stamp is the same as in a. Scale bar = 50um. d) In the first hour after injury and at 8 days after injury, monocytes and macrophages have a higher average speed than microglia. Each track was assessed for average speed. Tracking results were obtained from 228 microglia and 1601 monocytes/macrophages cells in three animals for each condition * P< 0.00001. e) In the first hour after injury and at 8 days after injury, monocytes and macrophages have a larger average displacement than microglia. Tracking is from 228 microglia and 1601 monocytes/macrophages cells in three animals for each condition * P<0.00001.
Figure 6. Axonal destruction occurs in response to contact only with Cx3cr1+/GFP blood-derived cells and not Cx3cr1+/GFP microglia
a) Example of contact between a Thy-1CFP/+ axon (yellow) and a Cx3cr1+/GFP blood-derived cell (green) resulting in axonal “blebbing” (white arrows) on day 5 after DCC injury. Blebbing occurred in two locations of the axon (yellow). b) Another example of a contact between a Thy-1YFP/+ axon (yellow) and a Cx3cr1+/GFP blood derived cell (green) resulting in axonal breakage (white arrows) on day 8 after DCC injury. c) Example of the interactions between microglia (green) and an axon (yellow) on day 8 after DCC injury. No change in axonal size or integrity was observed during an hour-long imaging session. Colors here are different than in A and B to allow easier identification of yellow axons. d) Quantification of contacts between axons and blood-derived macrophages, with steady numbers of contacts and destructive contacts between axons and monocytes/macrophages seen on days 2, 5 and 8. We observed a total of 232 contacts and 22 destructive contacts in 10 blood derived Cx3cr1+/GFP mice. e) Quantification of contacts between axons and resident microglia, with contacts but no destructive contacts seen on days 2, 5 and 8. We observed a total of 52 contacts and no destructive contacts in 11 resident Cx3cr1+/GFP mice.
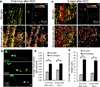
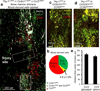
Figure 7. Very few macrophages are both Cx3cr1_+/GFP_ and Ccr2+/RFP positive. Resident microglia are primarily Cx3cr1_+/GFP_, while blood derived macrophages consist of populations of both Cx3cr1_+/GFP_ positive macrophages and Ccr2+/RFP positive macrophages.. Axonal position after dieback is not affected by CCR2 deficiency
Since all instances of axon thinning and breaking in in vivo imaging were in contact with a Cx3cr1+/GFP cell, we wanted to see if these cells are part of the same or different population than CCR2+ macrophages. a) Compiled immunofluorescence images of a DCC lesion (solid white box) 5 days after injury in fixed, sectioned tissue in a Thy-1+/YFP (white) × Cx3cr1+/GFP (green) × Ccr2+/RFP (red) triple-reporter mouse. A caudal region containing degenerating axons is marked with a dotted white box. Cx3cr1_+/GFP_ and Ccr2+/RFP cells are found around the lesion. Scale bar is 200um. b) Quantification of Cx3cr1+/GFP (green) and Ccr2+/RFP (red) blood derived cells found in the lesion in chimeric mice. Quantification is performed in three slices in each of three mice. c) A triple transgenic animal 5 days after DCC expressing Thy-1YFP, Cx3cr1+/GFP and Ccr2RFP/+. Scale bar = 100um. d) A triple transgenic animal 5 days after DCC expressing Thy-1YFP, Cx3cr1+/GFP and Ccr2RFP/RFP. Scale bar = 100 um. e) Quantification of axon position from the center of the lesion in these animals as described in the methods. N=5 for each condition. P=0.10 using a one tailed t-test assuming samples of unequal variance. There is no difference between _Ccr2RFP/+_animals and Ccr2RFP/RFP animals, indicating that Ccr2 is also not singly responsible for axonal position after injury. Since we only observed destructive events with Cx3cr1+/GFP cells in time lapse imaging, and no difference in dieback is see in CCR2 deficient animals, we can infer that the Ccr2+/RFP cells, although present in the lesion, are unlikely to be responsible for these destructive events.
Similar articles
- Intravital imaging of axonal interactions with microglia and macrophages in a mouse dorsal column crush injury.
Evans TA, Barkauskas DS, Myers JT, Huang AY. Evans TA, et al. J Vis Exp. 2014 Nov 23;(93):e52228. doi: 10.3791/52228. J Vis Exp. 2014. PMID: 25489963 Free PMC article. - Expression pattern of Ccr2 and Cx3cr1 in inherited retinal degeneration.
Kohno H, Koso H, Okano K, Sundermeier TR, Saito S, Watanabe S, Tsuneoka H, Sakai T. Kohno H, et al. J Neuroinflammation. 2015 Oct 12;12:188. doi: 10.1186/s12974-015-0408-3. J Neuroinflammation. 2015. PMID: 26458944 Free PMC article. - The toll-like receptor 2 agonist Pam3CSK4 is neuroprotective after spinal cord injury.
Stivers NS, Pelisch N, Orem BC, Williams J, Nally JM, Stirling DP. Stivers NS, et al. Exp Neurol. 2017 Aug;294:1-11. doi: 10.1016/j.expneurol.2017.04.012. Epub 2017 Apr 23. Exp Neurol. 2017. PMID: 28445714 - Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.
Puntambekar SS, Saber M, Lamb BT, Kokiko-Cochran ON. Puntambekar SS, et al. Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review. - A view from the ending: Axonal dieback and regeneration following SCI.
Hill CE. Hill CE. Neurosci Lett. 2017 Jun 23;652:11-24. doi: 10.1016/j.neulet.2016.11.002. Epub 2016 Nov 5. Neurosci Lett. 2017. PMID: 27825985 Review.
Cited by
- P2Y12 expression and function in alternatively activated human microglia.
Moore CS, Ase AR, Kinsara A, Rao VT, Michell-Robinson M, Leong SY, Butovsky O, Ludwin SK, Séguéla P, Bar-Or A, Antel JP. Moore CS, et al. Neurol Neuroimmunol Neuroinflamm. 2015 Mar 19;2(2):e80. doi: 10.1212/NXI.0000000000000080. eCollection 2015 Apr. Neurol Neuroimmunol Neuroinflamm. 2015. PMID: 25821842 Free PMC article. - Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice.
Li Y, Ritzel RM, Khan N, Cao T, He J, Lei Z, Matyas JJ, Sabirzhanov B, Liu S, Li H, Stoica BA, Loane DJ, Faden AI, Wu J. Li Y, et al. Theranostics. 2020 Sep 14;10(25):11376-11403. doi: 10.7150/thno.49199. eCollection 2020. Theranostics. 2020. PMID: 33052221 Free PMC article. - CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages.
Lei F, Cui N, Zhou C, Chodosh J, Vavvas DG, Paschalis EI. Lei F, et al. Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23336-23338. doi: 10.1073/pnas.1922788117. Epub 2020 Sep 8. Proc Natl Acad Sci U S A. 2020. PMID: 32900927 Free PMC article. - Cavin-1 promotes M2 macrophages/microglia polarization via SOCS3.
Huang W, Hong Y, He W, Jiang L, Deng W, Peng B, Tang F, Shen C, Lan Q, Huang H, Zhong H, Lv J, Zeng S, Li M, OuYang Y, Liang J, Mo Z, Chen Q, Cui L, Zhang M, Xu F, Zhou Z. Huang W, et al. Inflamm Res. 2022 Apr;71(4):397-407. doi: 10.1007/s00011-022-01550-w. Epub 2022 Mar 11. Inflamm Res. 2022. PMID: 35275225 - Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury.
Qian Z, Chen H, Xia M, Chang J, Li X, Ye S, Wu S, Jiang S, Bao J, Wang B, Kong R, Zhang S, Zheng S, Cao X, Hong X. Qian Z, et al. Int J Biol Sci. 2022 Jan 16;18(4):1328-1346. doi: 10.7150/ijbs.68974. eCollection 2022. Int J Biol Sci. 2022. PMID: 35280691 Free PMC article.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. - PubMed
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature neuroscience. 2011;14:1142–1149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA154656/CA/NCI NIH HHS/United States
- R01 NS25713/NS/NINDS NIH HHS/United States
- 5T32EB7509/EB/NIBIB NIH HHS/United States
- T32 EB007509/EB/NIBIB NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- R01 NS025713/NS/NINDS NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical