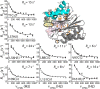Tracing an allosteric pathway regulating the activity of the HslV protease - PubMed (original) (raw)
Tracing an allosteric pathway regulating the activity of the HslV protease
Lichi Shi et al. Proc Natl Acad Sci U S A. 2014.
Abstract
The HslU-HslV complex functions as a bacterial proteasome, degrading substrate polypeptides to preserve cellular homeostasis. Here, we use methyl-Transverse Relaxation-Optimized Spectroscopy (TROSY) and highly deuterated, methyl-protonated samples to study the 230 kDa dodecameric HslV protease component that is structurally homologous to the stacked pair of β7-rings of the proteasome. Chemical shift assignments for over 95% of the methyl groups are reported. From the pH dependence of methyl chemical shifts, a pKa of 7.7 is measured for the amine group of the catalytic residue T1, confirming that it can act as a proton acceptor during the initial step in substrate proteolysis. Analyses involving a series of single site mutants in HslV, localized to HslU binding sites or regions undergoing significant changes on HslU binding, have identified hot spots whose perturbation leads to an allosteric pathway of propagated changes in structure and ultimately, substrate proteolysis efficiency. HslV plasticity is explored through methyl-TROSY (13)C relaxation dispersion experiments that are sensitive to millisecond timescale dynamics. The data support a dynamic coupling between residues involved in both HslU and substrate binding and residues localized to the active sites of HslV that facilitate the allostery between these distal sites. An important role for dynamics has also been observed in the archaeal proteasome, suggesting a more generally conserved role of motion in the function of these barrel-like protease structures.
Keywords: molecular machines; protein NMR; protein dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
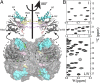
Fig. 1.
Near-complete I, L, M, T, and V methyl chemical shift assignments for HslV. (A) Surface model showing the dodecameric structure of HslV, comprising a pair of hexameric rings that are aligned coaxially (9) (Protein Data Bank ID code 1G3K) with the front four subunits removed to show the proteolytic chamber. All monomers of the hexamer are equivalent. Shown in Upper are ribbon diagrams for a pair of monomers, corresponding to the two front-facing subunits of the surface model, related to each other by a 180° rotation about an axis of sixfold symmetry. Helices are labeled H1, H2 (highlighted in cyan), H2A, H3, and H4, with catalytic residue T1 in red. Parts of the substrate binding pocket (S1, S3, and S3′ from an adjacent molecule) are also indicated. (B) Regions of 13C,1H HMQC spectra of [U-2H; Ileδ1-13CH3; Leu,Val-13CH3/12CD3; Met-13CH3]– or [U-2H; Ileδ1-13CH3; Met-13CH3; Thr-13CH3]–labeled HslV (18.8T and 40 °C).
Fig. 2.
Measurement of active site T1 amine pKa establishes the first step of the catalytic mechanism. (A) Superposition of selected regions of HMQC spectra as a function of pH, focusing on residues T1, T2, and I3. (B) Chemical shift titration data were fit on a per-residue basis to a model that involved ionization of two groups, which is described in the text. The 13C and 1H shifts of a given methyl group were fit simultaneously (e.g., T1γ2). Duplicate measurements at pH 7.5 and pH 10 were used to estimate errors in measured chemical shifts; the error bar is within the size of each dot. (C) Ribbon diagram of a region of a single HlsV monomer highlighting the active site (Protein Data Bank ID code 1G3K). Residues that are critical for the proposed peptidase activity are shown in ball-and-stick representation (
SI Appendix
). (D) pH profile of HslV peptidase efficiency based on hydrolysis rates of the substrate Z-GGL-AMC (_N_-carbobenzoxy-Gly-Gly-Leu-amido-4-methylcoumarin; 40 °C).
Fig. 3.
An allosteric pathway regulating the function of HslV. (A) The structure of each HslV monomer is approximately symmetric about a pseudotwofold axis that runs through the center of the protein, which is indicated by the blue arrow. Shown in gray (cyan for H1/H2) is the structure of an HslV monomer using the same orientation as in Fig. 1, right subunit, whereas the structure obtained by applying a 180° rotation about the symmetry axis (blue arrow) is indicated in gold. The positions of some of the mutations used are indicated. (B–E) Chemical shift changes relative to the WT protein are indicated in bar chart form and color-coded on the structure. Each methyl-containing residue is depicted in stick representation, and those methyls that undergo changes in shift ≥25 Hz are highlighted in orange. The mean chemical shift change (±1 SD) is indicated, excluding methyl groups ≤ 8 Å from the site of mutation. Note that the L88I mutation (H2) shows large changes in chemical shifts, whereas the mutation involving the symmetry-related position I159V shows little change. Where changes are observed, they extend from helices H1/H2 to regions surrounding the active site and/or substrate binding sites (S1, S3, and S3′) as indicated.
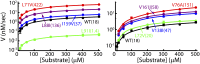
Fig. 4.
Peptidase assay for the HslV mutants considered. A fluorogenic peptide Z-GGL-AMC was used as a substrate. The _V_–substrate curves were fitted to a simple Michaelis–Menten equation (solid lines) as indicated. Note that mutations showing large chemical shift perturbations (Fig. 3 and
SI Appendix, Fig. S6
) also give rise to an order of magnitude change in activity (either increase or decrease), whereas mutants (for example, I159V) producing small chemical shift changes have little effect on activity. Values of k cat /K m (M−1s−1) are indicated. The values, including errors, are WT = 18 ± 2; L71V = 422 ± 8; L72V = 20 ± 4; V76A = 151 ± 10; L88I = 136 ± 5; L91I = 1.40 ± 0.03; V138I = 47 ± 5; I159V = 37 ± 8; and V161I = 58 ± 2.
Fig. 5.
Representative single quantum 1H or 13C-1H multiple quantum CPMG relaxation dispersion profiles (_R_2,eff vs. _ν_CPMG) for WT HslV (18.8T and 40 °C). Methyl-containing side chains are highlighted on the ribbon diagram and color-coded in orange in the case where R ex > 5 s−1.
Similar articles
- Structural alteration in the pore motif of the bacterial 20S proteasome homolog HslV leads to uncontrolled protein degradation.
Park E, Lee JW, Yoo HM, Ha BH, An JY, Jeon YJ, Seol JH, Eom SH, Chung CH. Park E, et al. J Mol Biol. 2013 Aug 23;425(16):2940-54. doi: 10.1016/j.jmb.2013.05.011. Epub 2013 May 21. J Mol Biol. 2013. PMID: 23707406 - Binding of MG132 or deletion of the Thr active sites in HslV subunits increases the affinity of HslV protease for HslU ATPase and makes this interaction nucleotide-independent.
Park E, Lee JW, Eom SH, Seol JH, Chung CH. Park E, et al. J Biol Chem. 2008 Nov 28;283(48):33258-66. doi: 10.1074/jbc.M805411200. Epub 2008 Oct 6. J Biol Chem. 2008. PMID: 18838376 Free PMC article. - HslVU ATP-dependent protease utilizes maximally six among twelve threonine active sites during proteolysis.
Lee JW, Park E, Jeong MS, Jeon YJ, Eom SH, Seol JH, Chung CH. Lee JW, et al. J Biol Chem. 2009 Nov 27;284(48):33475-84. doi: 10.1074/jbc.M109.045807. Epub 2009 Oct 1. J Biol Chem. 2009. PMID: 19801685 Free PMC article. - Dynamics of the ClpP serine protease: a model for self-compartmentalized proteases.
Liu K, Ologbenla A, Houry WA. Liu K, et al. Crit Rev Biochem Mol Biol. 2014 Sep-Oct;49(5):400-12. doi: 10.3109/10409238.2014.925421. Epub 2014 Jun 10. Crit Rev Biochem Mol Biol. 2014. PMID: 24915503 Review. - ClpP: a structurally dynamic protease regulated by AAA+ proteins.
Alexopoulos JA, Guarné A, Ortega J. Alexopoulos JA, et al. J Struct Biol. 2012 Aug;179(2):202-10. doi: 10.1016/j.jsb.2012.05.003. Epub 2012 May 14. J Struct Biol. 2012. PMID: 22595189 Review.
Cited by
- Chemical-Shift Perturbations Reflect Bile Acid Binding to Norovirus Coat Protein: Recognition Comes in Different Flavors.
Creutznacher R, Schulze E, Wallmann G, Peters T, Stein M, Mallagaray A. Creutznacher R, et al. Chembiochem. 2020 Apr 1;21(7):1007-1021. doi: 10.1002/cbic.201900572. Epub 2019 Dec 5. Chembiochem. 2020. PMID: 31644826 Free PMC article. - Disulfide-Bond-Induced Structural Frustration and Dynamic Disorder in a Peroxiredoxin from MAS NMR.
Troussicot L, Vallet A, Molin M, Burmann BM, Schanda P. Troussicot L, et al. J Am Chem Soc. 2023 May 17;145(19):10700-10711. doi: 10.1021/jacs.3c01200. Epub 2023 May 4. J Am Chem Soc. 2023. PMID: 37140345 Free PMC article. - The Context-Dependence of Mutations: A Linkage of Formalisms.
Poelwijk FJ, Krishna V, Ranganathan R. Poelwijk FJ, et al. PLoS Comput Biol. 2016 Jun 23;12(6):e1004771. doi: 10.1371/journal.pcbi.1004771. eCollection 2016 Jun. PLoS Comput Biol. 2016. PMID: 27337695 Free PMC article. No abstract available. - Entropy redistribution controls allostery in a metalloregulatory protein.
Capdevila DA, Braymer JJ, Edmonds KA, Wu H, Giedroc DP. Capdevila DA, et al. Proc Natl Acad Sci U S A. 2017 Apr 25;114(17):4424-4429. doi: 10.1073/pnas.1620665114. Epub 2017 Mar 27. Proc Natl Acad Sci U S A. 2017. PMID: 28348247 Free PMC article. - Ravens, New Caledonian crows and jackdaws parallel great apes in motor self-regulation despite smaller brains.
Kabadayi C, Taylor LA, von Bayern AM, Osvath M. Kabadayi C, et al. R Soc Open Sci. 2016 Apr 20;3(4):160104. doi: 10.1098/rsos.160104. eCollection 2016 Apr. R Soc Open Sci. 2016. PMID: 27152224 Free PMC article.
References
- Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. - PubMed
- Baumeister W, Walz J, Zühl F, Seemüller E. The proteasome: Paradigm of a self-compartmentalizing protease. Cell. 1998;92(3):367–380. - PubMed
- Löwe J, et al. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science. 1995;268(5210):533–539. - PubMed
- Bochtler M, Ditzel L, Groll M, Hartmann C, Huber R. The proteasome. Annu Rev Biophys Biomol Struct. 1999;28:295–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases