Microglia development and function - PubMed (original) (raw)
Review
Microglia development and function
Debasis Nayak et al. Annu Rev Immunol. 2014.
Abstract
Proper development and function of the mammalian central nervous system (CNS) depend critically on the activity of parenchymal sentinels referred to as microglia. Although microglia were first described as ramified brain-resident phagocytes, research conducted over the past century has expanded considerably upon this narrow view and ascribed many functions to these dynamic CNS inhabitants. Microglia are now considered among the most versatile cells in the body, possessing the capacity to morphologically and functionally adapt to their ever-changing surroundings. Even in a resting state, the processes of microglia are highly dynamic and perpetually scan the CNS. Microglia are in fact vital participants in CNS homeostasis, and dysregulation of these sentinels can give rise to neurological disease. In this review, we discuss the exciting developments in our understanding of microglial biology, from their developmental origin to their participation in CNS homeostasis and pathophysiological states such as neuropsychiatric disorders, neurodegeneration, sterile injury responses, and infectious diseases. We also delve into the world of microglial dynamics recently uncovered using real-time imaging techniques.
Figures
Figure 1
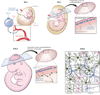
Microglial origin and development. Recent studies have shown that microglia are ontologically distinct from bone marrow–derived monocytes/macrophages found in peripheral tissues. Microglia are instead derived from primitive macrophages that emanate from the embryonic yolk sac during development (prior to embryonic day 8.5, E8.5) and enter the brain rudiment via the circulatory system. These progenitors surround the neuroepithelium of the developing brain around E9.5 and one day later enter the neuroepithelium and begin to colonize the CNS parenchyma. Microglia at this stage of development have an amoeboid rather than a ramified morphology. Microglia become completely ramified throughout the brain by postnatal day 28. Development and survival of microglia are critically dependent on several factors, including the transcription factor PU.1 as well as CSF1R.
Figure 2
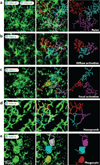
Microglia activation following CNS injury and infection. Illustration of (a) focal and (b) diffuse microglia activation resulting from sterile brain injury and viral infection, respectively. Microglia in the naive CNS are highly ramified and continuously scan the parenchyma as part of their homeostatic program (see Figure 3; Supplemental Videos a1 and a2). (a) Upon focal brain injury that induces necrotic cell death, damaged tissue and surrounding astrocytes release extracellular ATP, which triggers activation of specific purinergic receptors expressed by microglia. For example, microglial detection of ATP via P2Y12R and P2X4R (two purinergic receptors) induces the extension of processes toward the injury epicenter and the concurrent retraction of all other processes (see Figure 3; Supplemental Video c). The extent of tissue injury likely dictates how quickly microglia convert to a phagocyte and participate in lesion cleanup. For example, detection of UDP via P2Y6R causes microglia to invest their cellular material into a single phagocytic process, which is followed by retraction of the soma into this process (see Figure 3; Supplemental Video e). The resultant phagocyte then participates in the cleanup of cellular debris. (b) Following CNS infection with a noncytopathic virus (e.g., LCMV), microglia processes become shorter and less branched but maintain a ramified structure (see Figure 3; Supplemental Video b). Microglia in this morphological state can release proinflammatory cytokines and antivirals, engage in antigen presentation, and facilitate the arrival of peripheral innate/adaptive immune cells.
Figure 3
Morphological transformations of microglia in vivo. Representative 50 µm maximum projections of microglia (green) in the xy plane were captured through the surgically thinned skulls of CX3CR1gfp/+ mice by two-photon laser scanning microscopy. The processes of individual microglia were labeled in different colors using the filament tracer feature in Imaris. (a) Naive microglia possess multiple, highly ramified processes extending in all directions (see Supplemental Videos a1 and a2). (b) Upon diffuse activation induced by CNS viral infection with LCMV, microglial processes become shorter and less complex than naive microglia but retain a ramified structure (see Supplemental Video b). (c) Upon focal activation induced by a meningeal compression injury, microglia directionally extend multiple processes toward the focal site of injury, concurrently retracting processes in all other directions (see Supplemental Video c). (d) In response to damage at or above the glial limitans, astrocytes recruit microglial processes that line the borders between individual cells to form a continuous, honeycomb-like network composed of processes from numerous individual microglia (see Supplemental Video d). (e) In response to necrotic cell death, microglia become phagocytic and sometimes motile by first extending a single large circular extension toward the injured cell while retracting all other processes. The microglia soma is eventually pulled into the phagocytic extension along the thin connecting process (see Supplemental Video e). To view these videos, access this article on the Annual Reviews website at
and click on the above panels. These videos are also provided in the Supplemental Material
Similar articles
- Microglia Biology: One Century of Evolving Concepts.
Prinz M, Jung S, Priller J. Prinz M, et al. Cell. 2019 Oct 3;179(2):292-311. doi: 10.1016/j.cell.2019.08.053. Cell. 2019. PMID: 31585077 Review. - Microglia as therapeutic target in central nervous system disorders.
Fatoba O, Itokazu T, Yamashita T. Fatoba O, et al. J Pharmacol Sci. 2020 Nov;144(3):102-118. doi: 10.1016/j.jphs.2020.07.004. Epub 2020 Aug 15. J Pharmacol Sci. 2020. PMID: 32921391 Review. - [The Role of Microglia in the Homeostasis of the Central Nervous System and Neuroinflammation].
Gogoleva VS, Drutskaya MS, Atretkhany KS. Gogoleva VS, et al. Mol Biol (Mosk). 2019 Sep-Oct;53(5):790-798. doi: 10.1134/S0026898419050057. Mol Biol (Mosk). 2019. PMID: 31661478 Review. Russian. - Microglia across the lifespan: from origin to function in brain development, plasticity and cognition.
Tay TL, Savage JC, Hui CW, Bisht K, Tremblay MÈ. Tay TL, et al. J Physiol. 2017 Mar 15;595(6):1929-1945. doi: 10.1113/JP272134. Epub 2016 May 29. J Physiol. 2017. PMID: 27104646 Free PMC article. Review. - Age-related alterations in the dynamic behavior of microglia.
Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Damani MR, et al. Aging Cell. 2011 Apr;10(2):263-76. doi: 10.1111/j.1474-9726.2010.00660.x. Epub 2010 Dec 29. Aging Cell. 2011. PMID: 21108733 Free PMC article.
Cited by
- Gut Microbiota to Microglia: Microbiome Influences Neurodevelopment in the CNS.
Bettag J, Goldenberg D, Carter J, Morfin S, Borsotti A, Fox J, ReVeal M, Natrop D, Gosser D, Kolli S, Jain AK. Bettag J, et al. Children (Basel). 2023 Oct 31;10(11):1767. doi: 10.3390/children10111767. Children (Basel). 2023. PMID: 38002858 Free PMC article. Review. - The Use of Identified Hypoxia-related Genes to Generate Models for Predicting the Prognosis of Cerebral Ischemia‒reperfusion Injury and Developing Treatment Strategies.
Sun K, Li H, Dong Y, Cao L, Li D, Li J, Zhang M, Yan D, Yang B. Sun K, et al. Mol Neurobiol. 2024 Sep 4. doi: 10.1007/s12035-024-04433-9. Online ahead of print. Mol Neurobiol. 2024. PMID: 39230867 - Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury.
Mierzwa AJ, Marion CM, Sullivan GM, McDaniel DP, Armstrong RC. Mierzwa AJ, et al. J Neuropathol Exp Neurol. 2015 Mar;74(3):218-32. doi: 10.1097/NEN.0000000000000165. J Neuropathol Exp Neurol. 2015. PMID: 25668562 Free PMC article. - Effect of General Anesthetic Agents on Microglia.
Yang Y, Hang W, Li J, Liu T, Hu Y, Fang F, Yan D, McQuillan PM, Wang M, Hu Z. Yang Y, et al. Aging Dis. 2024 May 7;15(3):1308-1328. doi: 10.14336/AD.2023.1108. Aging Dis. 2024. PMID: 37962460 Free PMC article. Review. - The involvement of α-synucleinopathy in the disruption of microglial homeostasis contributes to the pathogenesis of Parkinson's disease.
Miao Y, Meng H. Miao Y, et al. Cell Commun Signal. 2024 Jan 12;22(1):31. doi: 10.1186/s12964-023-01402-y. Cell Commun Signal. 2024. PMID: 38216911 Free PMC article. Review.
References
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. - PubMed
- Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013;61:121–127. - PubMed
- Del Rio-Hortega P. Microglia. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System. New York: P.B. Hoeber; 1937. pp. 481–534.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous