Conventional transmission electron microscopy - PubMed (original) (raw)
Conventional transmission electron microscopy
Mark Winey et al. Mol Biol Cell. 2014 Feb.
Abstract
Researchers have used transmission electron microscopy (TEM) to make contributions to cell biology for well over 50 years, and TEM continues to be an important technology in our field. We briefly present for the neophyte the components of a TEM-based study, beginning with sample preparation through imaging of the samples. We point out the limitations of TEM and issues to be considered during experimental design. Advanced electron microscopy techniques are listed as well. Finally, we point potential new users of TEM to resources to help launch their project.
Figures
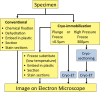
FIGURE 1:
A brief flowchart showing the work to be done with different types of sample preparation for conventional electron microscopy (yellow background). The advanced cryo-EM techniques are shown with a blue background. For immuno-EM, the samples can be stained before embedding (pre-embedding staining) or the sections can be stained (post-embedding staining).
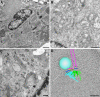
FIGURE 2:
Cell structure as visualized by transmission electron microscopy. (A) Actin-myosin cytoskeleton revealed in a cultured cardiomyocyte prepared by conventional chemical fixation. Bar, 1 µm (B) Cytoplasmic organelles in a mouse macrophage prepared by conventional chemical fixation. Bar, 700 nm (C) Golgi membranes in a cultured 3T3 cell prepared by high-pressure freezing and freeze substitution. Bar, 200 nm. (D) Three-dimensional tomographic model of a forming mitotic spindle from budding yeast. Bar, 200 nm.
Similar articles
- Osmium tetroxide and ruthenium tetroxide are complementary reagents for the preparation of epidermal samples for transmission electron microscopy.
Swartzendruber DC, Burnett IH, Wertz PW, Madison KC, Squier CA. Swartzendruber DC, et al. J Invest Dermatol. 1995 Mar;104(3):417-20. doi: 10.1111/1523-1747.ep12665909. J Invest Dermatol. 1995. PMID: 7861011 - New versatile staining reagents for biological transmission electron microscopy that substitute for uranyl acetate.
Nakakoshi M, Nishioka H, Katayama E. Nakakoshi M, et al. J Electron Microsc (Tokyo). 2011 Dec;60(6):401-7. doi: 10.1093/jmicro/dfr084. J Electron Microsc (Tokyo). 2011. PMID: 22146677 - A preparation of bacterial outer membrane with osmium tetroxide and uranyl acetate co-stain enables improved structural determination by transmission electron microscopy.
Sheikh A, Zechmann B, Sayes CM, Taube JH, Greathouse KL. Sheikh A, et al. Microscopy (Oxf). 2023 Nov 24;72(6):515-519. doi: 10.1093/jmicro/dfad027. Microscopy (Oxf). 2023. PMID: 37148329 Free PMC article. - Quantification of structures and gold labeling in transmission electron microscopy.
Lucocq J. Lucocq J. Methods Cell Biol. 2008;88:59-82. doi: 10.1016/S0091-679X(08)00404-4. Methods Cell Biol. 2008. PMID: 18617028 Review. No abstract available. - Cryogenic transmission electron microscopy: aqueous suspensions of nanoscale objects.
Burrows ND, Penn RL. Burrows ND, et al. Microsc Microanal. 2013 Dec;19(6):1542-53. doi: 10.1017/S1431927613013354. Epub 2013 Sep 4. Microsc Microanal. 2013. PMID: 24001937 Review.
Cited by
- Combinatorial Antimicrobial Efficacy and Mechanism of Linalool Against Clinically Relevant Klebsiella pneumoniae.
Yang SK, Yusoff K, Ajat M, Wee CY, Yap PS, Lim SH, Lai KS. Yang SK, et al. Front Microbiol. 2021 Mar 17;12:635016. doi: 10.3389/fmicb.2021.635016. eCollection 2021. Front Microbiol. 2021. PMID: 33815320 Free PMC article. - Correlative Imaging to Detect Rare HIV Reservoirs and Associated Damage in Tissues.
Valdebenito S, Ajasin D, Prideaux B, Eugenin EA. Valdebenito S, et al. Methods Mol Biol. 2024;2807:93-110. doi: 10.1007/978-1-0716-3862-0_7. Methods Mol Biol. 2024. PMID: 38743223 - Multiscale Electron Microscopy for the Study of Viral Replication Organelles.
Wolff G, Bárcena M. Wolff G, et al. Viruses. 2021 Jan 28;13(2):197. doi: 10.3390/v13020197. Viruses. 2021. PMID: 33525547 Free PMC article. Review. - TDP-43 and RNA form amyloid-like myo-granules in regenerating muscle.
Vogler TO, Wheeler JR, Nguyen ED, Hughes MP, Britson KA, Lester E, Rao B, Betta ND, Whitney ON, Ewachiw TE, Gomes E, Shorter J, Lloyd TE, Eisenberg DS, Taylor JP, Johnson AM, Olwin BB, Parker R. Vogler TO, et al. Nature. 2018 Nov;563(7732):508-513. doi: 10.1038/s41586-018-0665-2. Epub 2018 Oct 31. Nature. 2018. PMID: 30464263 Free PMC article. - Designing Gold Nanoparticles for Precise Glioma Treatment: Challenges and Alternatives.
Lansangan C, Khoobchandani M, Jain R, Rudensky S, Perry CC, Patil R. Lansangan C, et al. Materials (Basel). 2024 Mar 1;17(5):1153. doi: 10.3390/ma17051153. Materials (Basel). 2024. PMID: 38473623 Free PMC article. Review.
References
- Bouchet-Marquis C, Hoenger A. Cryo-electron tomography on vitrified sections: a critical analysis of benefits and limitations for structural cell biology. Micron. 2011;42:152–162. - PubMed
- Bozzola JJ, Russell LD. Electron Biology Principles and Techniques for Biologists. Sudbury, MA: Jones & Bartlett Learning; 1999.
- Dahl R, Staehelin LA. High-pressure freezing for the preservation of biological structure: theory and practice. J Electron Microsc Tech. 1989;13:165–174. - PubMed
- Gilkey JC, Staehelin LA. Advances in ultrarapid freezing for the preservation of cellular ultrastructure. J Electron Microsc Tech. 1986;3:177–210.
Publication types
MeSH terms
Substances
Grants and funding
- GM074746/GM/NIGMS NIH HHS/United States
- P41 GM103431/GM/NIGMS NIH HHS/United States
- R01 GM051312/GM/NIGMS NIH HHS/United States
- R01 GM074746/GM/NIGMS NIH HHS/United States
- 8P41GM103431/GM/NIGMS NIH HHS/United States
- GM51312/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources