The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women - PubMed (original) (raw)
doi: 10.1186/2049-2618-2-4.
Sonia S Hassan, Pawel Gajer, Adi L Tarca, Douglas W Fadrosh, Lorraine Nikita, Marisa Galuppi, Ronald F Lamont, Piya Chaemsaithong, Jezid Miranda, Tinnakorn Chaiworapongsa, Jacques Ravel
Affiliations
- PMID: 24484853
- PMCID: PMC3916806
- DOI: 10.1186/2049-2618-2-4
The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women
Roberto Romero et al. Microbiome. 2014.
Erratum in
- Correction: The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women.
Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. Romero R, et al. Microbiome. 2014 Apr 15;2(1):10. doi: 10.1186/2049-2618-2-10. Microbiome. 2014. PMID: 24735933 Free PMC article. No abstract available.
Abstract
Background: This study was undertaken to characterize the vaginal microbiota throughout normal human pregnancy using sequence-based techniques. We compared the vaginal microbial composition of non-pregnant patients with a group of pregnant women who delivered at term.
Results: A retrospective case-control longitudinal study was designed and included non-pregnant women (n = 32) and pregnant women who delivered at term (38 to 42 weeks) without complications (n = 22). Serial samples of vaginal fluid were collected from both non-pregnant and pregnant patients. A 16S rRNA gene sequence-based survey was conducted using pyrosequencing to characterize the structure and stability of the vaginal microbiota. Linear mixed effects models and generalized estimating equations were used to identify the phylotypes whose relative abundance was different between the two study groups. The vaginal microbiota of normal pregnant women was different from that of non-pregnant women (higher abundance of Lactobacillus vaginalis, L. crispatus, L. gasseri and L. jensenii and lower abundance of 22 other phylotypes in pregnant women). Bacterial community state type (CST) IV-B or CST IV-A characterized by high relative abundance of species of genus Atopobium as well as the presence of Prevotella, Sneathia, Gardnerella, Ruminococcaceae, Parvimonas, Mobiluncus and other taxa previously shown to be associated with bacterial vaginosis were less frequent in normal pregnancy. The stability of the vaginal microbiota of pregnant women was higher than that of non-pregnant women; however, during normal pregnancy, bacterial communities shift almost exclusively from one CST dominated by Lactobacillus spp. to another CST dominated by Lactobacillus spp.
Conclusion: We report the first longitudinal study of the vaginal microbiota in normal pregnancy. Differences in the composition and stability of the microbial community between pregnant and non-pregnant women were observed. Lactobacillus spp. were the predominant members of the microbial community in normal pregnancy. These results can serve as the basis to study the relationship between the vaginal microbiome and adverse pregnancy outcomes.
Figures
Figure 1
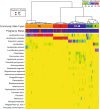
Heatmap of percentage abundance of microbial taxa found in the vaginal microbial communities of 22 normal pregnant women who delivered at term and 32 non-pregnant women sampled longitudinally. Ward linkage hierarchical clustering of Jensen-Shannon metric identified five community state types (CST I, II, III, IV-A and IV-B). The upper color bar shows the five community state types while the lower color bar shows the pregnancy status of each sample (NP: Non Pregnant; TD: Term Delivery).
Figure 2
Profiles of community state types for pregnant women who delivered at term as a function of gestational age. Gestational age at delivery is indicated by blue solid circles.
Figure 3
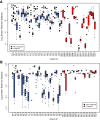
Estimates of community change over time in non-pregnant and pregnant women who delivered at term. (A) Jensen-Shannon distances between each community state and the mean community state for each subject. The larger the distance, the higher the instability of the microbial community within a subject. The mean within-subject log Jensen-Shannon distance of pregnant women was significantly lower than that for non-pregnant women (difference in means -0.473 log units; that is, 1.6-fold lower Jensen-Shannon distance, P < 0.001). (B) Jensen-Shannon distance between each community state and the mean community state of all samples assigned to CST IV-A and CST IV-B. The higher the distance, the less frequently a community enters CST IV-A or CST IV-B. The mean log Jensen-Shannon distance of pregnant women was significantly higher than that for non-pregnant women (difference in means 0.13 log units; that is, 1.14-fold, P < 0.001).
Similar articles
- Vaginal Microbiome Composition in Early Pregnancy and Risk of Spontaneous Preterm and Early Term Birth Among African American Women.
Dunlop AL, Satten GA, Hu YJ, Knight AK, Hill CC, Wright ML, Smith AK, Read TD, Pearce BD, Corwin EJ. Dunlop AL, et al. Front Cell Infect Microbiol. 2021 Apr 29;11:641005. doi: 10.3389/fcimb.2021.641005. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33996627 Free PMC article. - Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women.
Brotman RM, Bradford LL, Conrad M, Gajer P, Ault K, Peralta L, Forney LJ, Carlton JM, Abdo Z, Ravel J. Brotman RM, et al. Sex Transm Dis. 2012 Oct;39(10):807-12. doi: 10.1097/OLQ.0b013e3182631c79. Sex Transm Dis. 2012. PMID: 23007708 Free PMC article. - Vaginal host immune-microbiome interactions in a cohort of primarily African-American women who ultimately underwent spontaneous preterm birth or delivered at term.
Florova V, Romero R, Tarca AL, Galaz J, Motomura K, Ahmad MM, Hsu CD, Hsu R, Tong A, Ravel J, Theis KR, Gomez-Lopez N. Florova V, et al. Cytokine. 2021 Jan;137:155316. doi: 10.1016/j.cyto.2020.155316. Epub 2020 Oct 7. Cytokine. 2021. PMID: 33032107 Free PMC article. - The composition and stability of the vaginal microbiome of healthy women.
Alhabardi SM, Edris S, Bahieldin A, Al-Hindi RR. Alhabardi SM, et al. J Pak Med Assoc. 2021 Aug;71(8):2045-2051. doi: 10.47391/JPMA.1465. J Pak Med Assoc. 2021. PMID: 34418027 Review. - Vaginal microbiome.
Buchta V. Buchta V. Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English.
Cited by
- Determinants of Vaginal Microbiota Composition.
Moosa Y, Kwon D, de Oliveira T, Wong EB. Moosa Y, et al. Front Cell Infect Microbiol. 2020 Sep 2;10:467. doi: 10.3389/fcimb.2020.00467. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32984081 Free PMC article. Review. - Vaginal Microbiota Evaluation and Lactobacilli Quantification by qPCR in Pregnant and Non-pregnant Women: A Pilot Study.
Pacha-Herrera D, Vasco G, Cruz-Betancourt C, Galarza JM, Barragán V, Machado A. Pacha-Herrera D, et al. Front Cell Infect Microbiol. 2020 Jun 19;10:303. doi: 10.3389/fcimb.2020.00303. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32637367 Free PMC article. - The Central Nervous System and the Gut Microbiome.
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. Sharon G, et al. Cell. 2016 Nov 3;167(4):915-932. doi: 10.1016/j.cell.2016.10.027. Cell. 2016. PMID: 27814521 Free PMC article. Review. - A pilot study using unique targeted testing of the urogenital microbiome has potential as a predictive test during IVF for implantation outcome.
Evans GE, Mahajan V, Wakeman S, Slatter T, Ponnampalam AP, Anderson TP, Sarwar M, Evans JJ. Evans GE, et al. Arch Gynecol Obstet. 2023 Jun;307(6):1957-1967. doi: 10.1007/s00404-023-06987-w. Epub 2023 Mar 11. Arch Gynecol Obstet. 2023. PMID: 36905424 Free PMC article. - Vaginal microbiota in pregnancy: Role in induction of labor and seeding the neonate''s microbiota?
Kervinen K, Kalliala I, Glazer-Livson S, Virtanen S, Nieminen P, Salonen A. Kervinen K, et al. J Biosci. 2019 Oct;44(5):116. J Biosci. 2019. PMID: 31719225 Review.
References
- Döderlein A. Das Scheidensekret und seine Bedeutung fuerdas Puerperalfieber. Die Arten des Scheidensekretes. 1892;11:699.
- Thomas S. Döderlein Bacillus: lactobacillus acidophilus. J Infect Dis. 1928;43:218–227.
- Rogosa M, Sharpe ME. Species differentiation of human vaginal lactobacilli. J Gen Microbiol. 1960;23:197–201. - PubMed
- Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856–872. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous