Optimal translational termination requires C4 lysyl hydroxylation of eRF1 - PubMed (original) (raw)
. 2014 Feb 20;53(4):645-54.
doi: 10.1016/j.molcel.2013.12.028. Epub 2014 Jan 30.
Atsushi Yamamoto 1, Sarah E Wilkins 2, Elizaveta Sokolova 3, Luke A Yates 4, Martin Münzel 2, Pooja Singh 1, Richard J Hopkinson 2, Roman Fischer 5, Matthew E Cockman 1, Jake Shelley 1, David C Trudgian 6, Johannes Schödel 7, James S O McCullagh 2, Wei Ge 2, Benedikt M Kessler 5, Robert J Gilbert 4, Ludmila Y Frolova 3, Elena Alkalaeva 3, Peter J Ratcliffe 1, Christopher J Schofield 2, Mathew L Coleman 8
Affiliations
- PMID: 24486019
- PMCID: PMC3991326
- DOI: 10.1016/j.molcel.2013.12.028
Optimal translational termination requires C4 lysyl hydroxylation of eRF1
Tianshu Feng et al. Mol Cell. 2014.
Abstract
Efficient stop codon recognition and peptidyl-tRNA hydrolysis are essential in order to terminate translational elongation and maintain protein sequence fidelity. Eukaryotic translational termination is mediated by a release factor complex that includes eukaryotic release factor 1 (eRF1) and eRF3. The N terminus of eRF1 contains highly conserved sequence motifs that couple stop codon recognition at the ribosomal A site to peptidyl-tRNA hydrolysis. We reveal that Jumonji domain-containing 4 (Jmjd4), a 2-oxoglutarate- and Fe(II)-dependent oxygenase, catalyzes carbon 4 (C4) lysyl hydroxylation of eRF1. This posttranslational modification takes place at an invariant lysine within the eRF1 NIKS motif and is required for optimal translational termination efficiency. These findings further highlight the role of 2-oxoglutarate/Fe(II) oxygenases in fundamental cellular processes and provide additional evidence that ensuring fidelity of protein translation is a major role of hydroxylation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
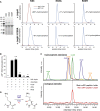
Jmjd4 Interacts with the eRF1/eRF3a Translational Termination Complex in an Activity-Dependent Manner (A) Protein sequences of human JmjC domains were aligned and shaded using Jalview. The Jmjd6 secondary structure, as defined by crystallographic analysis (Mantri et al., 2010), is indicated with α helices (cylinders) and β strands (arrows). The conserved double-stranded β helix core is in cyan (arrows). Triangles indicate residues binding Fe(II) (red) and 2OG (yellow). Mutation of the first Fe(II)-binding residue (His189 in Jmjd4) is predicted to inhibit activity. (B) Anti-FLAG immunoprecipitates of cell extracts from the indicated HEK293T cell lines were immunoblotted for endogenous eRF1 and eRF3a. Input (5%) = cell extract prior to immunoprecipitation (IP). eRF1 levels were quantified by densitometry analysis using NIH ImageJ. (C) HEK293T cell lines were immunostained for eRF1 (green) and FLAG-Jmjd4 (red). Nuclei were visualized with DAPI (blue). (D) Endogenous eRF1 and Jmjd4 interact. eRF1 was immunoprecipitated from HEK293T extracts prior to immunoblot for the indicated proteins. (E) Overexpressed Jmjd4 promotes hydroxylation of overexpressed eRF1 at K63. Left: Coomassie gel showing 5% input following anti-V5 purification of the V5-eRF1/HA-eRF3a complex from HEK293T cells overexpressing empty vector or FLAG-Jmjd4 (immunoblot bottom panel). The remainder of the sample (95%) was digested with Arg-C in-solution prior to LC-MS analyses. The chromatograms indicate the elution time and relative abundance of extracted ion masses corresponding to unhydroxylated (blue) and K63-hydroxylated (red) eRF148–65 ([M+H]3+; K63-H: m/z 646.68; K63-OH: m/z 652.00) in the absence (middle) and presence (right) of FLAG-Jmjd4. See also Figure S1.
Figure 2
Jmjd4 Catalyzes 2OG- and Fe(II)-Dependent C4 Lysyl Hydroxylation of eRF1 (A) K63 mutation prevents Jmjd4-dependent hydroxylation of eRF1. Left: Coomassie gel of partially purified recombinant eRF1 and mutants. Right: LC-MS extracted ion chromatograms (EICs) show wild-type and mutant eRF1 reacted with either wild-type (top row) or mutant H189A (bottom row) Jmjd4. ([M+H]2+; K63-H: m/z 698.842; K63-OH: m/z 706.840; K63R-H: m/z 712.846; K63R-OH: m/z 720.843; K63A-H: m/z 791.880; K63A-OH: m/z 799.878). (B) Jmjd4 is a 2OG/Fe(II)-dependent oxygenase. In vitro assays were performed in the presence or absence of the indicated cofactors and inhibitors. 2OG oxygenases are competitively inhibited by NOG, a nonhydrolysable form of 2OG. Data represent mean ± SEM. Statistical significance was evaluated by ANOVA followed by Dunett’s post hoc test, comparing all treatments to the reaction complemented with all cofactors (n.s., not significant; ∗∗p < 0.01). (C) Jmjd4 is a C4 lysyl hydroxylase. Bottom: a cyclic thioether-linked dimer of a 15-mer peptide containing eRF1 residues 57–70 was untreated (buffer, black) or Jmjd4 hydroxylated (red) prior to hydrolysis and LC-MS. Top: chromatography peaks observed in biological samples were identified with C3, C4, and/or C5 hydroxylysine standards. Note that two peaks are observed because each standard is a mixture of stereoisomers. See Figure S2 for NMR of standards and further validation of the C4 assignment. (D) Schematic of C4 lysyl hydroxylation catalyzed by Jmjd4. See also Figure S2.
Figure 3
Endogenous eRF1 K63 Hydroxylation Is Abundant, Ubiquitous, and Dependent on Jmjd4 Activity (A) HEK293T eRF1 was trypsinized prior to MS/MS. Spectra show a +16 Da mass shift in all detected y ion fragments (y2–y12) (blue) when comparing the upper (unhydroxylated) and lower (hydroxylated) panels. In contrast, the masses of detected b ion fragments (b2–b12) (red) are consistent with their predicted values, indicating K63 hydroxylation. This was confirmed with Arg-C proteolysis (Figure S3A). (B) LC-MS analyses of trypsinized eRF1 indicate abundant K63 hydroxylation in multiple cell types. EICs show relative abundance of unhydroxylated (blue) versus K63 hydroxylated (red) eRF151–63 ([M+H]2+; K63-H: m/z 698.842; K63-OH: m/z 706.840). (C) EICs demonstrate that eRF1 hydroxylation is dependent on Jmjd4 activity ([M+H]2+; K63-H: m/z 701.852; K63-OH: m/z 709.850). The masses are +3 Da relative to (B) due to SILAC (stable isotope labeling by/with amino acids in cell culture) with K+6. HeLa cells expressing empty vector (left), siRNA-resistant HA-Jmjd4 (middle), or siRNA-resistant HA-Jmjd4 H189A mRNAs (right) were transfected with control (top row) or Jmjd4 siRNA (bottom row) prior to SILAC labeling and LC-MS quantitation of hydroxylation in newly synthesized eRF1. Note that although hydroxylation of newly synthesized eRF1 is <15% following Jmjd4 siRNA, total eRF1 is ∼50% hydroxylated due to persisting eRF1 synthesized prior to siRNA (data not shown). (D) Cell extracts from (C) were immunoblotted for the indicated proteins. eRF1 levels were quantified by densitometry analysis. (E) Similar analyses in rodent tissues indicate that eRF1 hydroxylation is physiologically relevant and conserved. Rabbit reticulocyte lysate (RRL) and the indicated mouse tissues were diluted or homogenized in lysis buffer prior to eRF1 purification, trypsinolysis, and MS analyses. See also Figure S3.
Figure 4
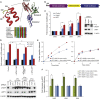
eRF1 K63 Hydroxylation Promotes Translational Termination Efficiency (A) Top: K63 is located at the apex of an α-helical extension within eRF1 domain 1 (Chimera and Protein Data Bank ID 1DT9) (Song et al., 2000). Bottom: alignment of the eRF1 NIKS region, indicating complete conservation of K63 across diverse species (Jalview). (B) A bicistronic reporter for measuring stop codon readthrough (Grentzmann et al., 1998) with luciferase cDNAs separated by a stop or sense codon (CAG) within a leaky termination sequence from tobacco mosaic virus. (C) Jmjd4 knockdown promotes stop codon readthrough. Left: HeLa cells were transfected with the reporters in (B) prior to siRNA (si) and dual luciferase assay. Note that K63 hydroxylation is ∼50% under similar knockdown conditions (see Figure 3 legend). Data represent mean ± SEM. Statistical significance was evaluated by ANOVA followed by Dunett’s post hoc test comparing Jmjd4 and eRF1 knockdown samples to control (∗∗p < 0.01). Right: immunoblot of cell extracts (n.s., nonspecific). (D) Jmjd4 activity is required for efficient translational termination. Top: stop codon readthrough assays were performed as in (C) using HeLa cell lines expressing empty vector, siRes-HA-Jmjd4, siRes-HA-Jmjd4 H189A, or siRes-HA-eRF1 mRNAs. Bottom: immunoblot of cell extracts. Data represent mean ± SEM. Comparisons across cell lines were made by ANOVA followed by Bonferroni post hoc test (∗∗p < 0.01). (E) K63 hydroxylation increases the catalytic efficiency of eRF1 in vitro. Unhydroxylated (post-Jmjd4-H189A treatment; blue) or hydroxylated (post-Jmjd4 treatment; red; ∼60% hydroxylation) wild-type (left) or K63R (right) eRF1 were added to pretermination ribosomal complexes before quantifying released radiolabelled peptides over time (shown is an example of data obtained with the UAA stop codon). Note that contaminating Jmjd4 was removed from eRF1 preparations (see Figure S4N). (F) Termination efficiencies (Kcat/KM × 104 [M−1s−1]) of wild-type (green) and K63R eRF1 (blue) at all three stop codons were calculated from initial rates and plotted as fold increase relative to Jmjd4 H189A-treated eRF1. Data represent mean ± SEM. Statistical significance was evaluated by paired two-tailed Student’s t test (∗∗p < 0.01). See also Figure S4.
References
- Alkalaeva E.Z., Pisarev A.V., Frolova L.Y., Kisselev L.L., Pestova T.V. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell. 2006;125:1125–1136. - PubMed
- Bidou L., Allamand V., Rousset J.P., Namy O. Sense from nonsense: therapies for premature stop codon diseases. Trends Mol. Med. 2012;18:679–688. - PubMed
- Coleman M.L., Ratcliffe P.J. Signalling cross talk of the HIF system: involvement of the FIH protein. Curr. Pharm. Des. 2009;15:3904–3907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 090532/WT_/Wellcome Trust/United Kingdom
- MR/K010816/1/MRC_/Medical Research Council/United Kingdom
- 090532/Z/09/Z/WT_/Wellcome Trust/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous